Chemical Bonding
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
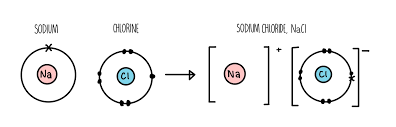
Ionic Bonding
The electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions), formed when electrons are transferred from one atom to another.
Typically occurs between metals and nonmetals.
Results in the formation of a crystalline lattice structure (NOT molecules).
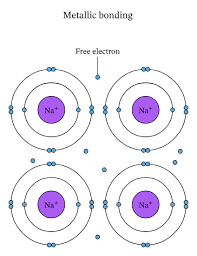
Metallic bonding
Chemical bonding in metals, where positively charged metal ions are surrounded by a “sea of delocalized electrons” that move freely throughout the metal lattice.
Explains metal properties:
Conductivity (electrons move freely)
Malleability and ductility (ions can slide past each other)
Luster (electrons reflect light)
Example: bonding in copper (Cu) or iron (Fe).
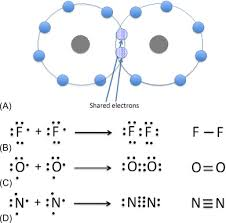
Covalent Bonding
Chemical bond formed when two atoms share one or more pairs of electrons to achieve a stable electron configuration.
Usually occurs between nonmetals.
Can be single, double, or triple bonds depending on the number of shared electron pairs.
Example: H₂O → each H shares 1 electron with O, forming two single covalent bonds.
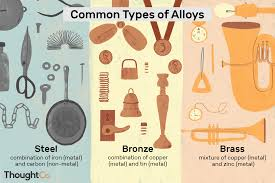
Alloys
Mixtures of elements where at least one element is a metal. Combinations of metals formed through metallic bonding with advanced metallic properties such as conductivity, luster, malleability, ductilibility, boiling points, and densities.
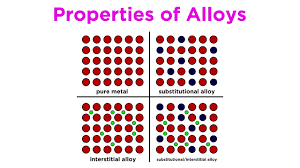
Interstitial Alloys
The different components have different atomic radii, causing the smaller component to slip in between the larger ones. This causes the alloy to become more rigid than substitutional alloys.
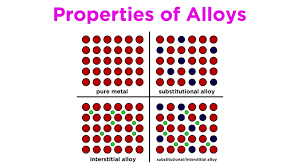
Substitutional Alloys
The different components have similar atomic radii, causing relatively similar densities due to the continuous pattern within the alloy. Increased malleability.
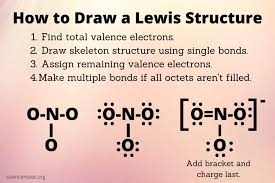
Lewis Structures
A type of simplified Bohr model to show the number of valence electrons an element has to simplify ionic and covalent bonding models.
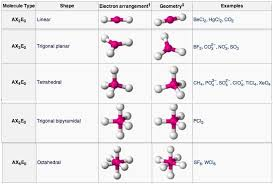
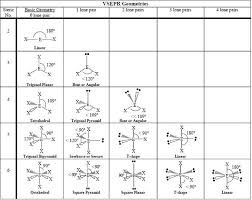
VSEPR
Valence Shell Electron Pair Repulsion Theory:
A model used to predict the shape of molecules based on the idea that electron domains (bonding and lone pairs) around a central atom repel each other and arrange themselves to minimize repulsion.
Electron domains include: single, double, triple bonds, and lone pairs.
Determines both electron geometry (all domains) and molecular shape (bonding domains only).
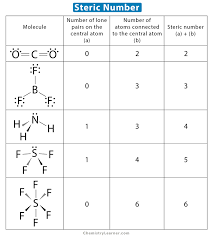
Steric Number
Indicates the number of electron domains when determining molecular geometry. Single, Double, and Triple covalent bonds all count as singular domains and loan pairs (set of 2 restricted and unbonding/ed electrons) attached to the center element count as one domain.
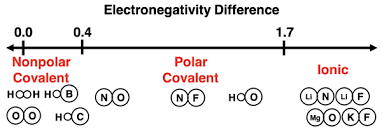
Polarity
Describes how electrons are unevenly shared in a covalent bond or distributed in a molecule, creating partial positive (δ⁺) and partial negative (δ⁻) charges.
Caused by differences in electronegativity between atoms.
Polar bond: electrons shared unequally.
Nonpolar bond: electrons shared equally.
Polar molecule: overall dipole exists
Nonpolar molecule: no net dipole
Can be calculated by finding the electronegativity difference or looking at a covalent-bonding diagram.
Cation
Metal result in ionic bonding, a positive ion (electrons<protons).
Anion
Nonmetal result in ionic bonding, a negative ion (electrons>protons).
Naming Ionic (and covalent) compounds
Cation (metal) + Anion (nonmetal) with the ending changed to -ide. Ex: Magnesium Chloride.
Also (sometimes) use prefixes to indicate the number of elements involved, though mosty for covalent naming.
Transitional metals may have different roman numerals after them depending on their charge, due to varying electron configurations. (Ex: Iron II)
Traditional ionic bonding naming system
In place of using roman numerals for the transitional metals when writing ionic bonding formulas/products. This system uses suffixes to denote the ending of the metal, causing you to work “backwards” to find the actual charge.
The -ic ending indicates a higher oxidation state/change
The -ous ending indicated a lower oxidation state/change
Cu2O = Cuprous oxide
CuO = Cupric oxide
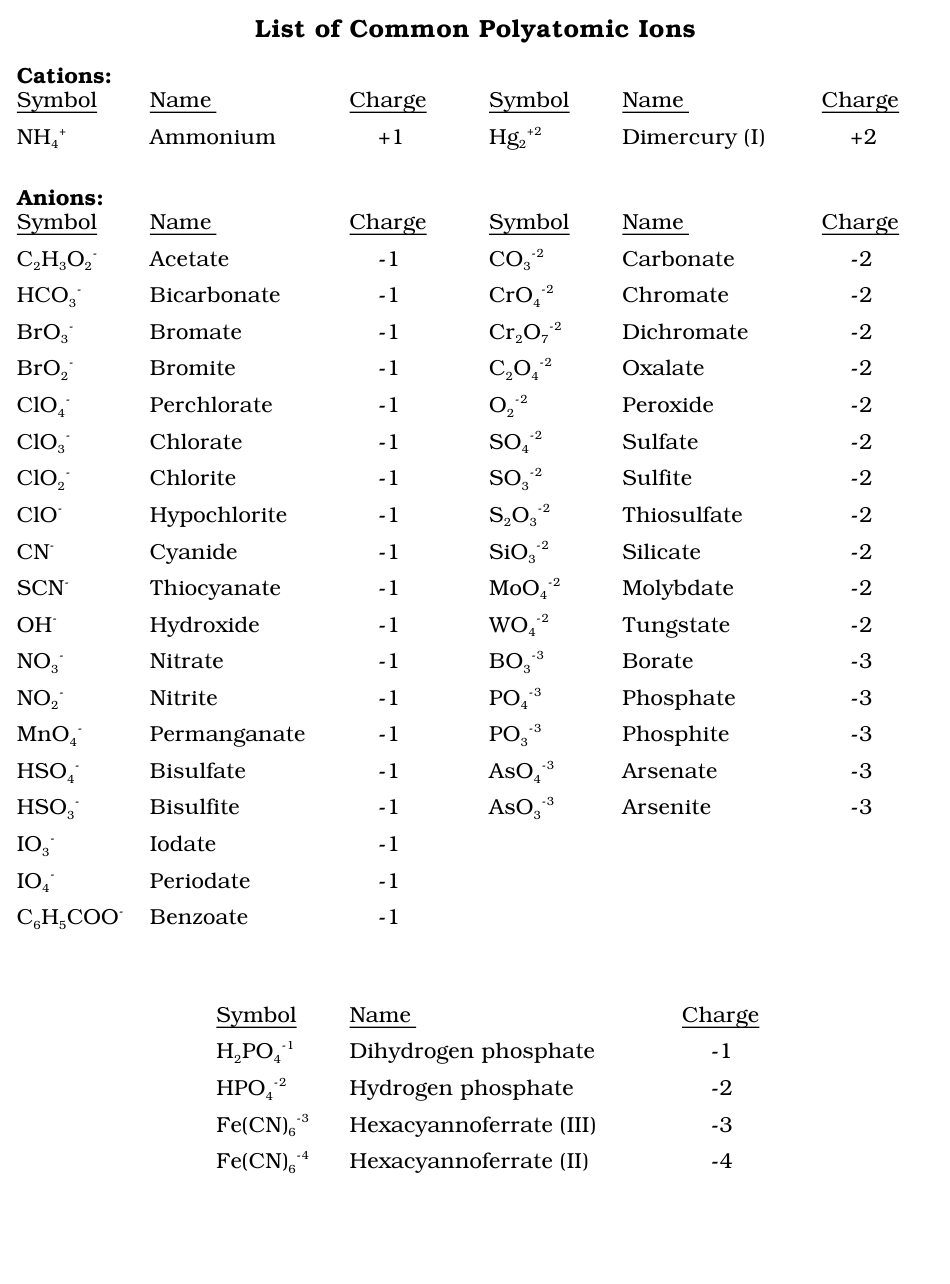
Polyatomic Ions
Compounds that are ions that must be treated as a single unit in ionic bonding. These use parenthesis to make the compound parts and subscripts easier to read. subscripts must be distributed if uses multiple polyatomic bonds.
Octet rule
Used in both Ionic and covalent bonding. Making the element a stable isotope by sharing/transferring enough electrons to fill the last (valence) shell, typically to 8 electrons.
Molecular Geometry
The three-dimensional shape of a molecule determined by the arrangement of atoms around the central atom.
Depends on bonding domains and lone pairs on the central atom.
Determined using VSEPR theory.
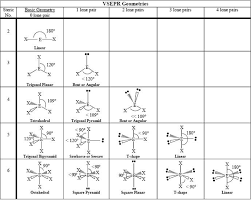
Symmetry
In a bonded compound indicated that the configuration stays the same in a 3-dimensional space.
Loan pairs affect symmetry and so do the types of atoms attached to the central atom.