Activation energy and measuring catalysed reaction rates
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
activation energy
the minimum amount of energy required for a chemical reaction to occur
what can the help of enzymes do to activation energy
interactions can be carried out with lower investment in a.e
what does energy have to reach before completing a reaction
transition state
transition state
intermediate state before being converted into products (e.g. bonds need to be broken/weakened in a substance)
how does an enzyme lower activation energy
weakening bonds so less energy is needed for the reaction to occur
how do we measure the rate of enzyme-catalysed reactions
record the amount of substarte has disappeared from a reaction mixture
alternative measure
amount of product that has accumulated in a unit of time
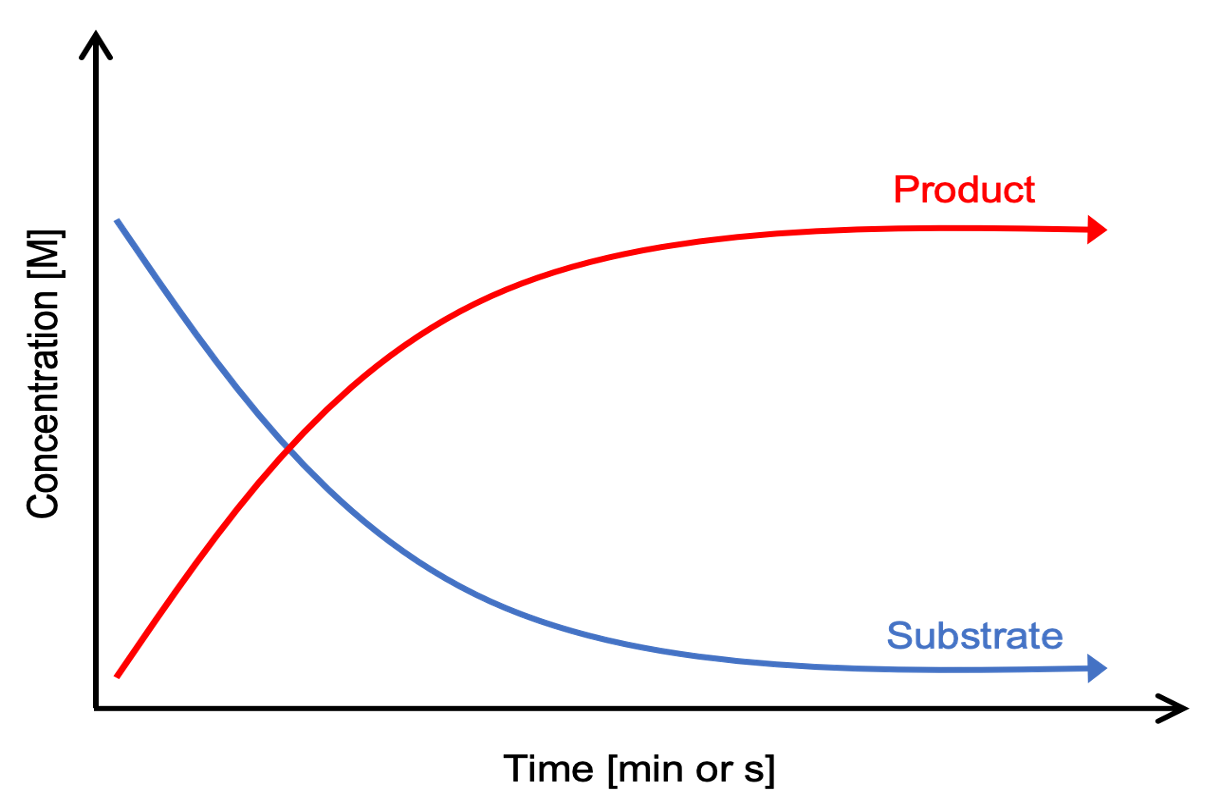
enzyme catalysed reaction (diagram)
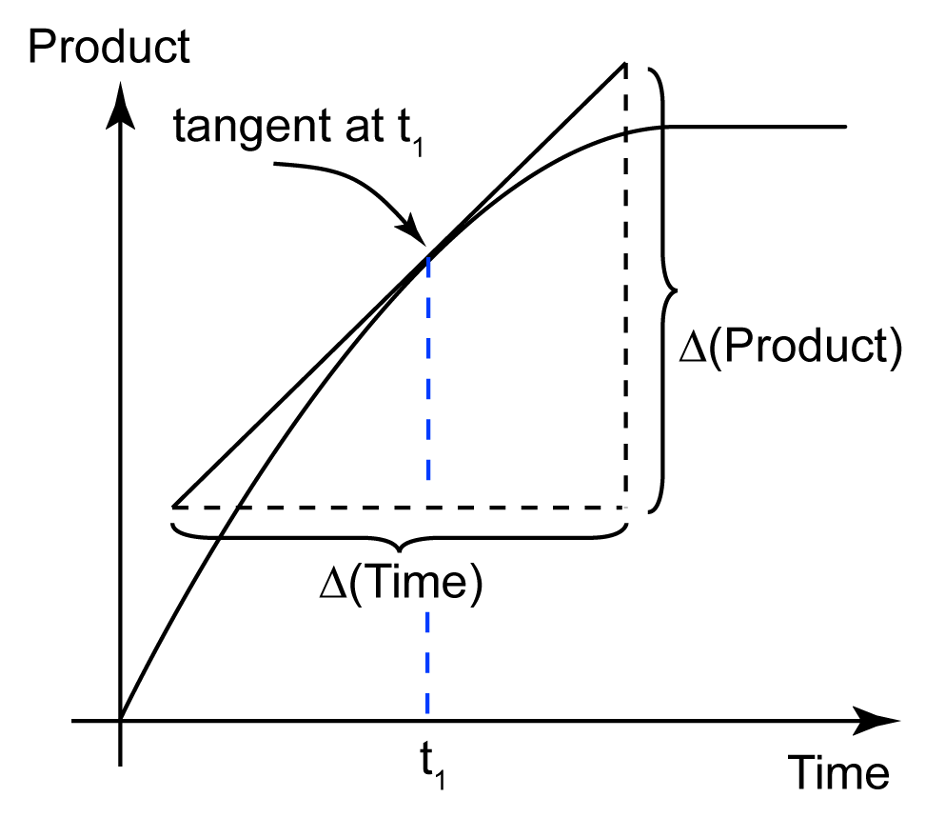
what is not constant in a reaction (graph)
the rate
how can the [instantaneous] rate [of reaction] be measured
draw a tangent to the curve at a specific time and divide the change in product by the change in time