OCR A 5.1.2 How Far?
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
reversible reaction
-where reaction can go back and for

for reaction
-initially reactants
-used up quickly but then slow as their conc drops
back reaction
-initially reactants are reformed slowly
-but then speed up as conc of products increases
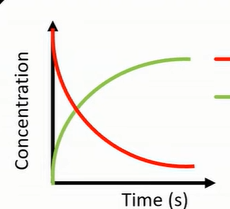
what is red, green
red: forward reaction; reactants
green: backward reaction; products
dynamic equilibrium
-rate of for reaction = rate of back reaction
-conc of each substance remains constant
-only occurs in closed system
Kc
-equilibrium constant
-can be worked out from the molar conc in a reaction
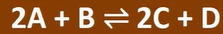
kc of this
products/reactants
molar value = power

homogeneous reaction
all reactants and products in same state
homogeneous reaction and Kc
all reactants and products included in final Kc expression
heterogeneous reaction
all reactants and products in dif states
total pressure
sum of all pressures of individual gasses (partial pressures)
partial pressures

mol fraction of gas
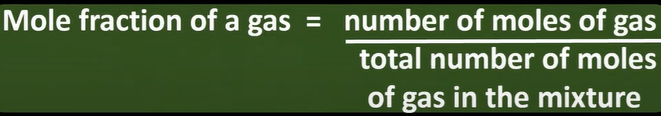
Kp
-equilibrium constant for equil reaction involving gases
-parital pressure used to calc
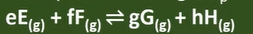
Kp expression
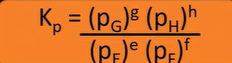
Kp and heterogeneous equilibria
when doing equation only use gages
le chatelier’s principle

which way will poe shift if pressure goes up
-shoft to side with fewestr gas particles
if delta H is negative
reaction is exo
increase temp in exo reaction: what happens to poe
-shifts to left to favour endo side
temp and Kp
if temp causes poe to shift to right Kp will increase
if temp change causes poe toshfit to left Kp will decrease
pressure and Kp
no effect
catalyst and Kp
no effect
conc and Kp
no effect