W1 L3: Embryonic Development
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
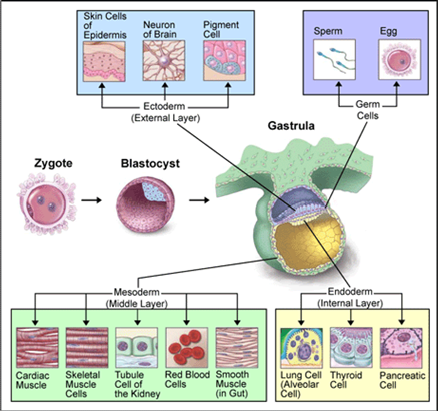
Cell types in Development
Epithelial cells
Mesenchymal cells
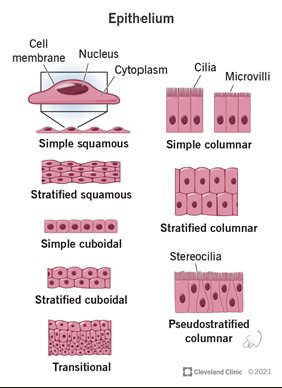
Epithelial cells
Epithelial cells are polarized cells with distinct apical (top) and basolateral (bottom) sides
Tightly connected to one another through structures like tight junction - maintain integrity and separation of cellular compartments
Line cavities and hollow structures throughout the body, such as the respiratory tract, digestive system, and blood vessels
Their primary role is to seal off underlying tissues, providing a protective barrier between the external environment and internal tissues
They create compartments, separating the "inside" (e.g., tissues and organs) from the "outside" (e.g., air, food, or pathogens).
Functions:
Protection: Prevents damage to internal tissues from physical, chemical, or biological factors
Barrier: Regulates the selective movement of substances in and out of compartments, ensuring proper exchange while maintaining defense
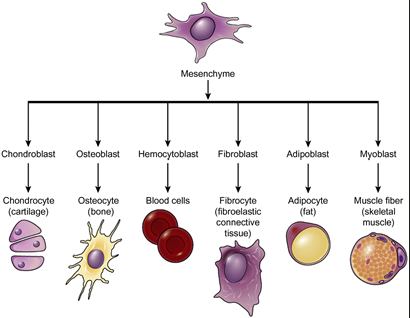
Mesenchymal cells
Mesenchymal cells are non-polarized cells that are loosely organized and highly migratory
Unlike epithelial cells, they lack tight junctions and can move through tissues
These cells are found in tissues that provide structural support, such as connective tissues, and play a key role in repair and regeneration
Functions:
Support: Contribute to the structural integrity and maintenance of tissues
Migration: Essential for tissue remodeling, wound healing, and development
Communication between cells in development
During embryonic development, epithelial and mesenchymal cells communicate and undergo dynamic transitions to shape tissues and organs:
Epithelial-Mesenchymal Transition (EMT):
Epithelial cells lose their polarity and tight junctions to become mesenchymal cells. This is critical for processes like tissue formation, wound healing, and cancer metastasis.
Mesenchymal-Epithelial Transition (MET):
Mesenchymal cells revert to epithelial cells, allowing the reorganization of tissues during development or repair.
These transitions ensure proper coordination between the "barrier" and "support" roles of epithelial and mesenchymal cells in both normal physiology and pathological conditions
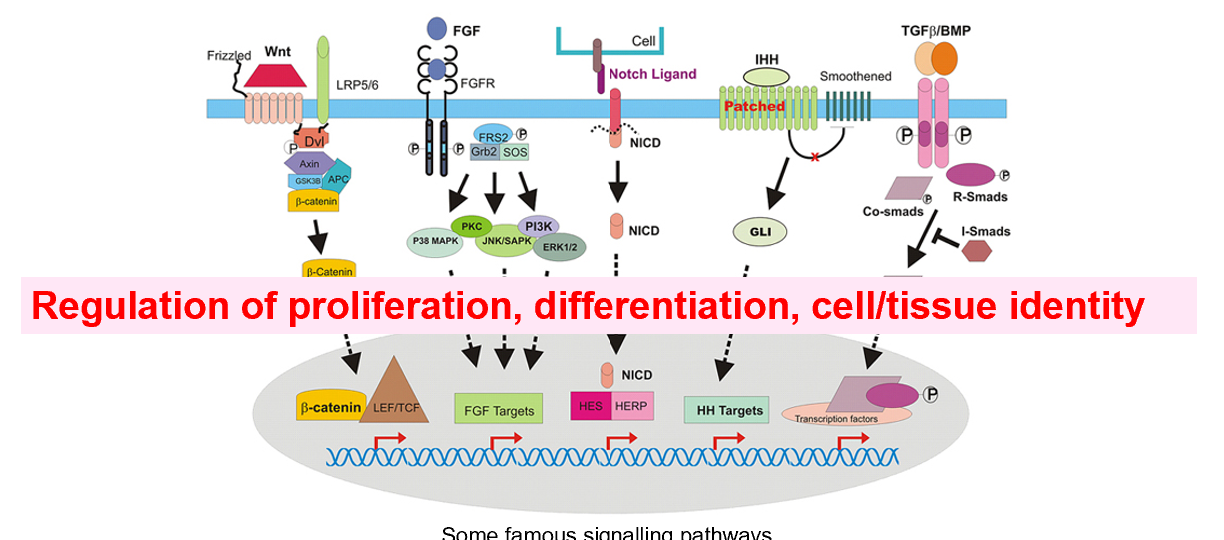
The neural crest
The neural crest is a unique group of embryonic cells in vertebrates that arises during early development
These cells are highly versatile and give rise to a diverse range of cell types and structures in the body
It plays a critical role in the formation of the nervous system, face, heart, and more
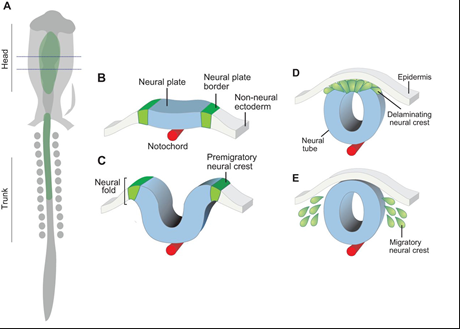
The neural crest - How do cells get specified
Medium levels of BMP (plus Wnt and FGF) >> induce neural crest
Activation of Pax3, Pax7, Zic1 (transcription factors)
Activation of Foxd3, Sox9, snail
Snail regulates the transition of epithelial to mesenchymal cell
>> EMT, Epithelial-Mesenchymal Transition
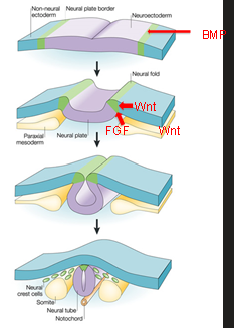
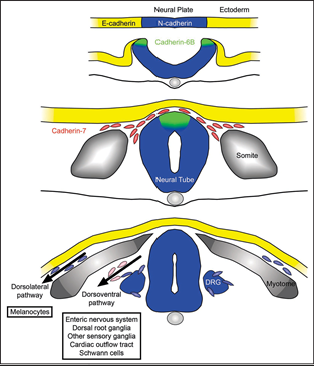
The neural crest - migration
E-Cadherin:
Found in surface ectoderm.
Maintains adhesion in non-migratory epithelial cells.
Downregulated during EMT to allow neural crest cells to separate.
N-Cadherin:
Expressed in neural tissue, including neural crest precursors.
Provides strong adhesion, keeping neural crest cells attached to the neural tube.
Downregulated during EMT to promote migration
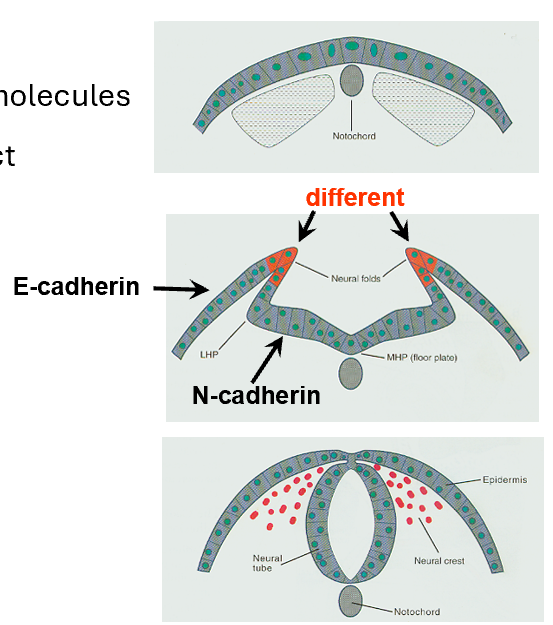
Cell-Cell Contact as a Signalling Mechanism
Cell adhesion molecules (CAM):
E-cadherin and N-cadherin are two key CAMs that mediate cell-cell adhesion and communication.
Specific roles of cadherins:
N-Cadherin: Expressed in neural tissue to maintain cell-cell adhesion within neural structures.
E-Cadherin: Found in the surface ectoderm, where it stabilizes epithelial structures.
Cadherin-6B: Expressed in neural crest cells and aids in their migration initiation.
Cadherin-7: Found in migratory neural crest (NC) cells, facilitating movement and pathway guidance.
These cadherins dynamically regulate adhesion to allow neural crest cells to detach, migrate, and reach their target tissues.
Cell differentiation in the neural crest
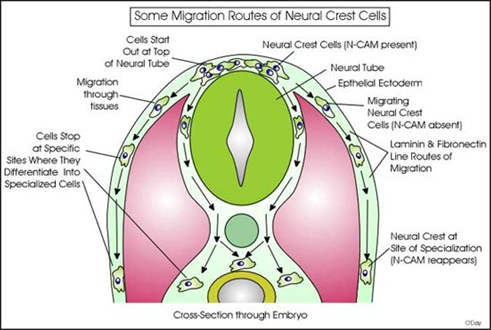
Neural crest cells migrate throughout the body:
Neural crest cells are multipotent cells that originate from the neural tube and travel to different parts of the embryo
As they migrate, they follow specific pathways and differentiate into specialized cell types at their target locations
Key roles of migration routes:
Cells start at the neural tube, migrate outward, and follow laminin and fibronectin (extracellular matrix components)
Migrating neural crest cells lose N-CAM adhesion temporarily, allowing movement, and regain it at their destination for differentiation
Derived structures: Neural crest cells form a wide variety of tissues, including parts of the enteric nervous system (in the intestine), dorsal root ganglia, Schwann cells, and melanocytes
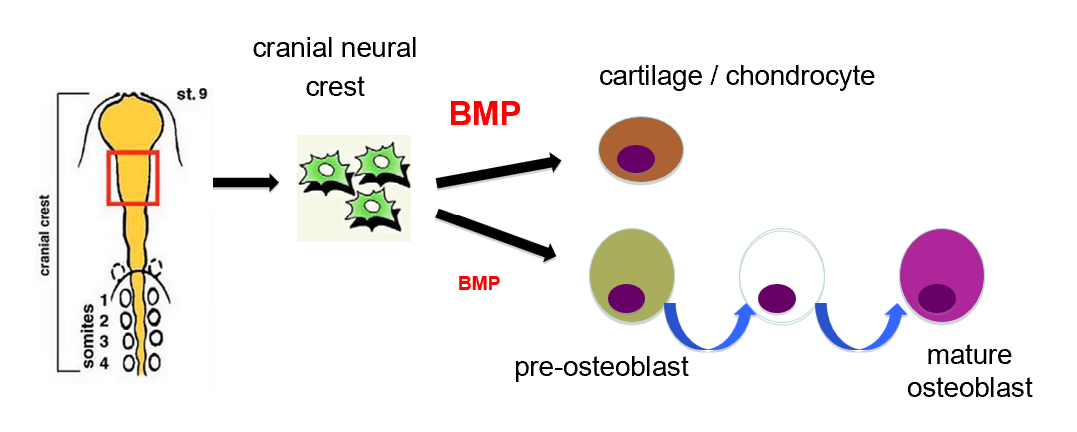
Cranial neural crest differentiation
Cranial Neural Crest
The cranial neural crest is a specialized population of neural crest cells originating from the cranial region of the neural tube
These cells are highly multipotent, meaning they can differentiate into various cell types, including:
Cartilage and chondrocytes (cartilage-producing cells)
Osteoblasts (bone-forming cells) involved in craniofacial development
Role of BMP (Bone Morphogenetic Protein)
BMP signaling is a key regulator of cranial neural crest differentiation. The levels of BMP determine the fate of these cells:
High BMP Levels:
Promote differentiation into cartilage/chondrocytes.
BMP enhances the expression of cartilage-specific genes, leading to the formation of chondrocytes, which produce extracellular matrix components like collagen.
Moderate BMP Levels:
Guide cranial neural crest cells into the osteoblast lineage (bone-forming pathway).
Neural crest cells first differentiate into pre-osteoblasts.
Through a series of maturation steps regulated by BMP, these pre-osteoblasts become mature osteoblasts, responsible for bone matrix production
Haematopoiesis
Haematopoiesis is the process through which blood cells are continuously generated and replenished throughout life
Haematopoietic Stem Cells
Hierarchically Primitive Cells: These are the most undifferentiated (primitive) cells at the top of the hierarchy, such as stem cells - high potential for self-renewal
They can also enter the G₀ phase (a resting state) or undergo apoptosis (programmed cell death) under certain conditions
Pathways of Development - Self-Renewal + Differentiation
Differentiation:
Multilineage Progenitors: Can give rise to multiple cell lineages but have limited potential compared to primitive stem cells
Unilineage Progenitors: Further specialized and committed to producing only one type of cell
Terminally Differentiated Cells: These are fully specialized cells with specific functions, such as: Neurons, Erythrocytes, Adipocytes, Osteocytes - cells then typically lose the ability to divide further
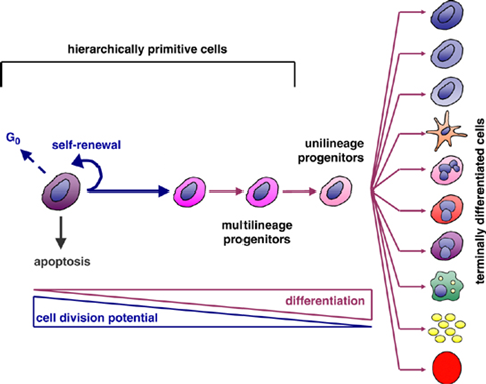
Relationship Between Division Potential and Differentiation
As cells progress from primitive stem cells to terminally differentiated cells:
Cell Division Potential Decreases:
Stem cells have the highest capacity for division, which diminishes as cells specialize
Differentiation Increases:
Cells become more specialized and less versatile as they differentiate
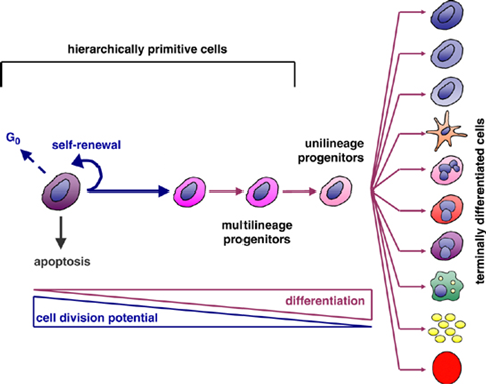
Haematopoietic stem cells (bone marrow)
Hematopoietic Stem Cells (HSCs) - Found in the bone marrow, multipotent stem cells capable of self-renewal and differentiation into all types of blood cells
Differentiation Pathways - HSCs differentiate into two major progenitor cell types
Lymphoid Progenitor Cells - Give rise to cells involved in the adaptive immune system
Myeloid Progenitor Cells - Give rise to cells involved in innate immunity, oxygen transport, and clotting
Bone Marrow Structure - The bone marrow is shown as the site of hematopoiesis, containing
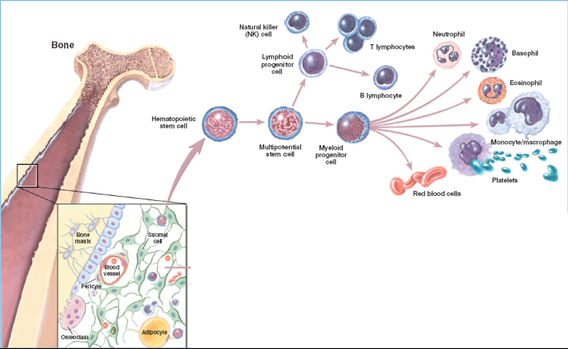
When does haematopoiesis start in the embryo
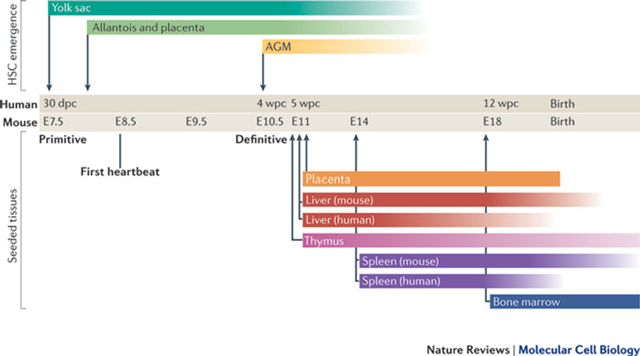
Primitive Hematopoiesis: Starts in the yolk sac at 30 dpc (human) or E7.5 (mouse) - Blood islands form, generating primitive erythrocytes (nucleated red blood cells) and a limited set of other blood cells like macrophages
Definitive Hematopoiesis: Starts in the AGM at 5 wpc (human) or E10.5 (mouse) and transitions to the liver, AGM (Aorta-Gonad-Mesonephros) region: This is where definitive hematopoietic stem cells (HSCs) first arise, HSCs from the AGM migrate to other sites, including: Placenta and Foetal Liver:
Bone Marrow Hematopoiesis: Begins around 12 wpc (human) or E18 (mouse) and continues after birth, HSCs settle in the bone marrow, which becomes the lifelong site of hematopoiesis
Stem cell niche
provides the Stem Cell with optimal environment
promotes self-renewal and blocks premature differentiation
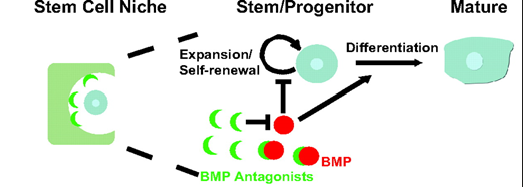
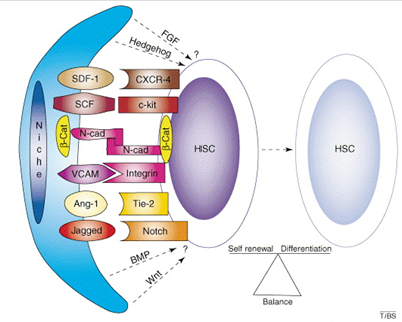
The Haematopoietic Stem Cell Niche
The hematopoietic stem cell niche is a specialized microenvironment in the bone marrow that regulates the behavior of hematopoietic stem cells, including their self-renewal, quiescence, proliferation, and differentiation
Osteoblasts and small vessels together form a dual niche system, crucial for the regulation and function of hematopoietic stem cells in the bone marrow
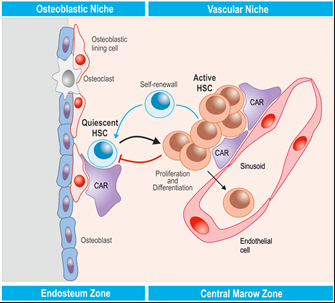
Regulation of Hematopoietic Stem Cells
Cytokines: Small proteins, such as SDF-1, act as signalling molecules that regulate HSC migration, retention, and activity in the niche, cytokines influence whether HSCs stay in a quiescent state or become activated
Growth Factors: Factors like SCF (stem cell factor) stimulate HSC proliferation and survival, maintain the stem cell population and prepare them for differentiation when needed
Ligands and Signaling Pathways: Notch and Jagged interactions: These regulate cell-fate decisions and promote HSC self-renewal, Wnt and BMP signaling pathways: Maintain HSCs in their undifferentiated state and control quiescence versus activation.
Cell-Surface and Adhesion Molecules: Molecules like N-cadherin and VCAM-1 facilitate HSC attachment to the niche, helping them remain in the microenvironment that sustains their functionality, Integrins and adhesion molecules also mediate interactions with stromal and endothelial cells.
Balance Between Self-Renewal and Differentiation:
The signals from the niche ensure a delicate equilibrium. If the balance tips too far toward self-renewal, the body may fail to generate sufficient blood cells. Conversely, excessive differentiation can deplete the stem cell pool, jeopardizing long-term hematopoiesis.
Through constant signaling, the niche helps HSCs respond to physiological demands, such as injury, infection, or normal cell turnover.