quiz #5: PDH complex, DNP
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
what is the PDH complex?
an enzyme complex that catalyzes the irreversible oxidative decarboxylation of pyruvate into acetyl coA to link glycolysis to the citric acid cycle
also generates NADH which will be used to make ATP using oxidative phosphorylation
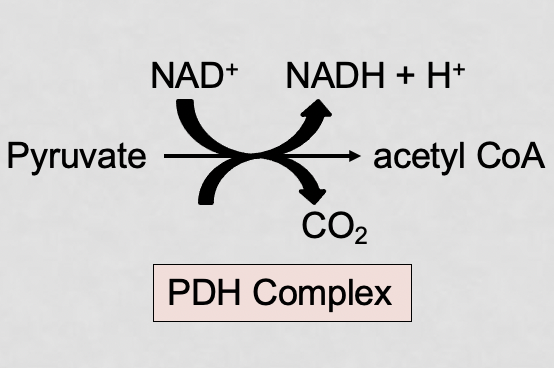
what are the 3 main enzymes of the PDH complex?
E1 (PDH), E2 (DLAT), E3 (DLD)
what does E1 do and what is its coenzyme?
catalyzes the decarboxylation of pyruvate, releasing CO2
coenzyme - TPP
what does E2 do and what is its coenzyme?
transfers the acetyl group to coenzyme A, forming acetyl coA, reduces lipoamide
coenzyme - lipoyllysine
what does E3 do and what is its coenzyme?
oxidizes lipoamide to regenerate it and and facilitates these electron to transfer to FAD → FADH2, these electrons are then given to NAD+ → NADH
coenzyme - FAD
what is the chemical reaction for the PDH complex?
pyruvate + coA + NAD+ → acetyl coA + CO2 + NADH + H+
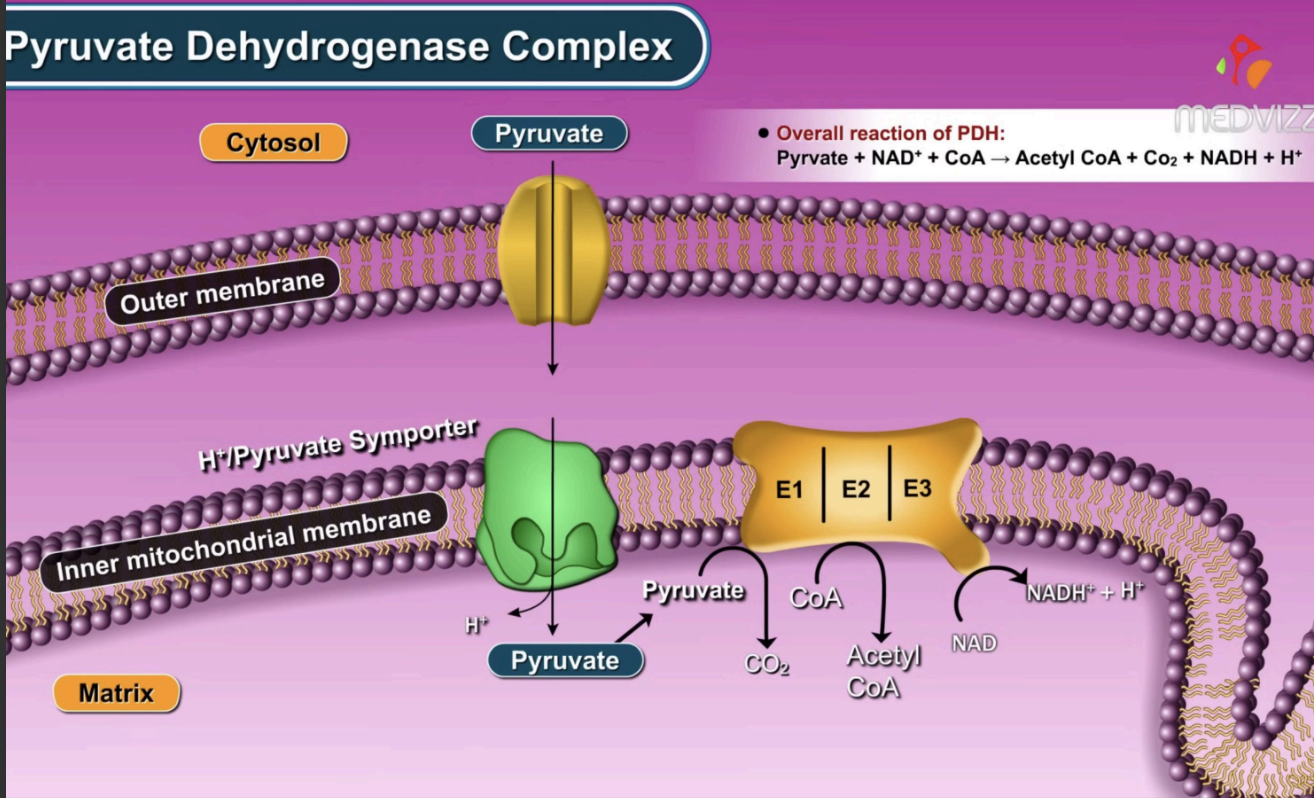
how does phosphorylation function in the PDH complex?
inactivation
PDH kinase (PDK) phosphoryates E1, inhibiting PDH
how does dephosphorylation function in the PDH complex?
activation
PDH phosphatase (PDP) removes the phosphate from E1, activating PDH
what are the allosteric inhibitors of the PDH complex?
ATP, acetyl coA, NADH
high energy charge
what are the allosteric activators of the PDH complex?
ADP, pyruvate, calcium ions
low energy charge
where does the PDH complex occur?
in the mitochondrial matrix
the synthesis of acetyl coA from pyruvate consists of what 3 steps?
decarboxylation of pyruvate
oxidation
transfer to coA
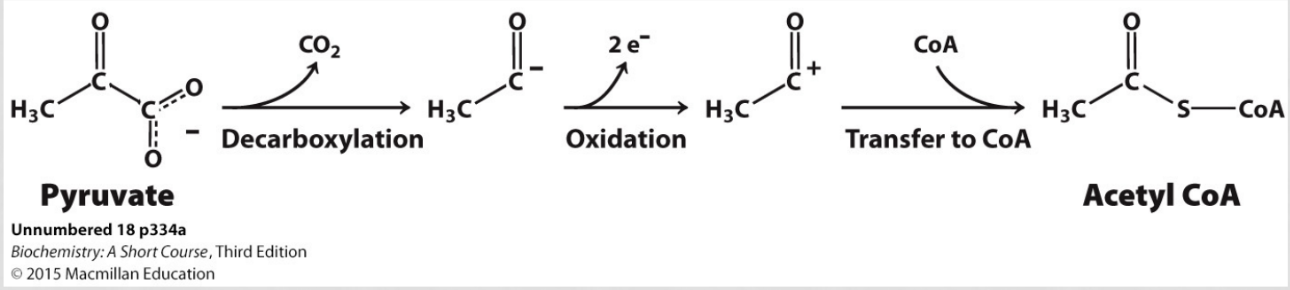
what is the difference between a catalytic coenzyme and a stoichiometric coenzyme?
catalytic - NOT consumed (TPP, lipoic acid, FAD)
stoichiometric - used as substrate (CoA, NAD+)
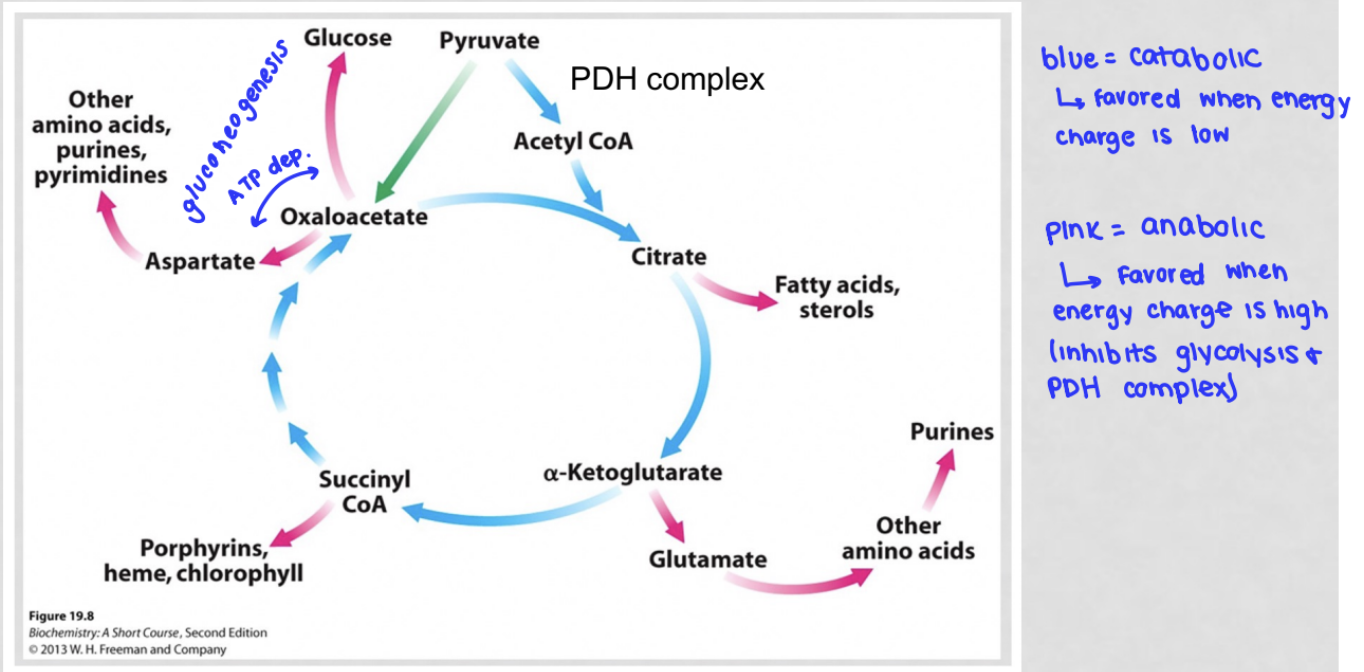
when is catabolism favored?
when energy charge is low
what happens to TCA cycle when energy charge is low?
catabolism favored
rate of TCA cycle increases, need for oxaloacetate increases
when is anabolism favored?
when energy charge is high
what happens to TCA cycle when energy charge is high?
anabolism is favored
anabolic pathways decrease the amount of oxaloacetate in the cell
what are the catabolic and anabolic parts of the citric acid cycle?
how many turns of TCA are there per glucose?
2
what happens in a PDH complex deficiency?
pyruvate: ↑ (not converted to acetyl coA)
lactate ↑
acetyl coA: ↓
ATP: ↓
since pyruvate is no longer able to be converted into acetyl coA in this reaction, the levels would accumulate in the cell. when pyruvate is not being used and builds up in the cell, it is converted to lactate by lactate dehydrogenase, causing an accumulation of lactate and and a decrease in pH of the surrounding tissue
since the PDH complex functions to form acetyl-coA to build produce energy in the cell, a malfunction in this complex would inhibit the series of steps to produce this end product, decreasing acetyl-coA in the cell. similarly, since this process is not occurring, NAD+ is not getting reduced, meaning ATP isn't being produced and the intracellular concentration will decrease
since atp is low, flux through glycolysis would increase to produce more pyruvate which is then converted to lactate
what are the goals of the citric acid cycle?
catabolic goals - degrade acetyl coA to generate NADH, FADH2, and some ATP
anabolic goals - intermediates serve as precursors to nucleic acid, amino acid, and fat synthesis
what are the fates of oxaloacetate?
enter TCA cycle (oxaloacetate + acetyl coA → citrate): recycles oxaloacetate
be used for gluconeogenesis (oxaloacetate → glucose): consumes oxaloacetate, decreasing its concentration
be converted to aspartate to convert other amino acids, purines, and pyrimidines
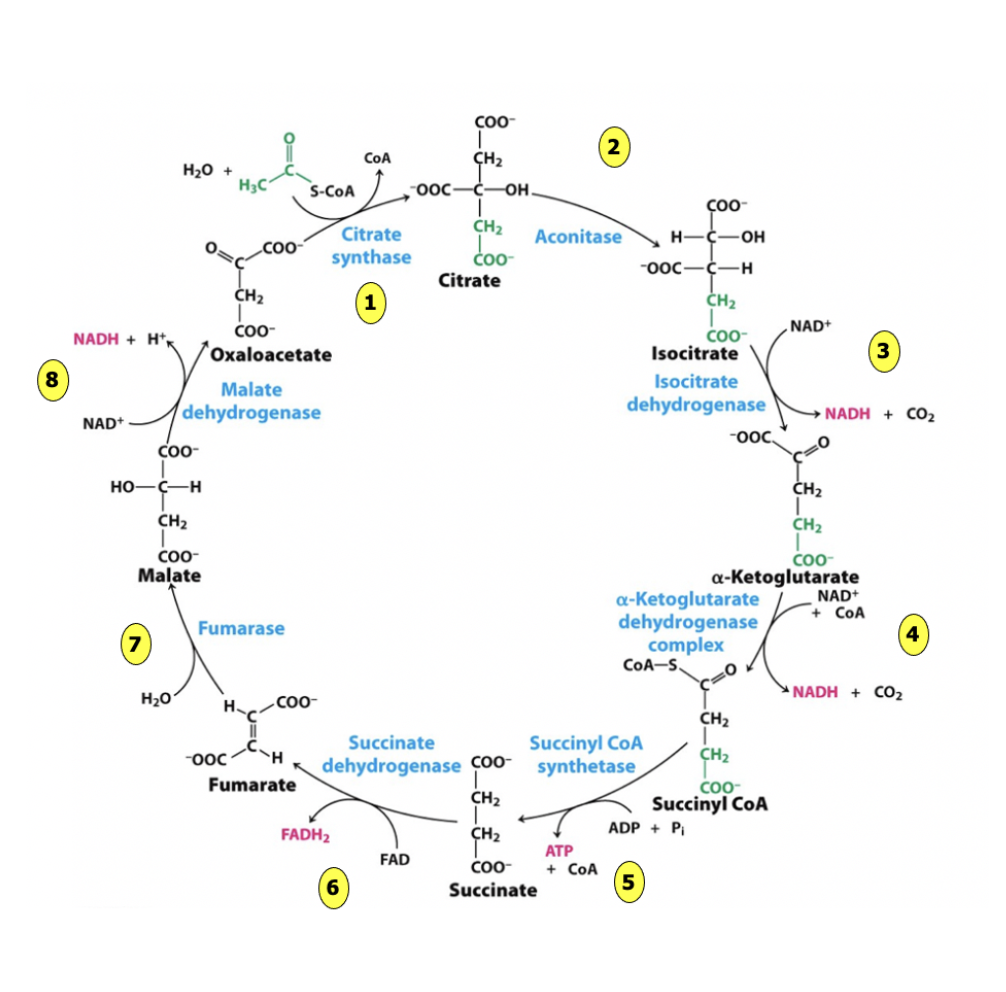
what are the net inputs of TCA cycle?
2 acetyl coA
6 NAD+
2 FAD
2 ADP + Pi
per glucose
what are the net outputs of TCA cycle?
4 CO2
6 NADH
2 FADH2
2 ATP
2 coA
per glucose
does oxaloacetate change in concentration?
no, it regenerates / recycles
is used in the first step but then subsequent steps remake it
pyruvate carboxylase is the only reaction that can replenish oxaloacetate!!!!
glycolysis occurs in the _ and net generates _ ATP and _ NADH per glucose
cytoplasm
2 ATP
2 NADH
the PDH reaction occurs in the _ and net generates _ ATP and _ NADH per glucose
mitochondrial matrix
0 ATP
2 NADH
TCA cycle occurs in the _ and net generates _ ATP and _ NADH and _ FADH2 per glucose
mitochondrial matrix
2 ATP
6 NADH
2 FADH2
glucose oxidation generates _ ATP via SLP, _ NADH and _ FADH2
4, 10, 2
an individual with a PDH complex deficiency can only perform what to oxidize glucose?
glycolysis
is the TCA cycle catabolic or anabolic?
both
what are the irreversible steps of TCA cycle?
1 ,3, 4
more energy is contained in a molecule that is fully _
reduced
what is reduction potential (E)?
measures the tendency of an oxidant to gain electrons or the tendency of a reductant to lose electrons in a half redox reaction
the higher the redox potential, the higher the affinity for electrons
strong oxidant = most +
strongest reductant = most -
what is the driving force of the ETC?
the electron transfer potential of NADH / FADH2 relative to O2
electrons are moving from a compound with lower affinity for electrons towards compounds with high affinities for electrons (O has greatest affinity)
what is the driving force of synthesis of ATP by oxidative phosphorylation?
proton motive force
what is the proton motive force?
an electrochemical gradient form of energy
what is the c-ring?
a component of ATP synthase that rotates with each H+ flux
what are some of the differences between oxidative phosphorylation and SLP?
OP requires O2 which is not directly used by ATP synthase but instead consumed by the ETC to generate the proton motive force
SLP requires certain substrate to provide phosphoryl transfer energy
OP only takes place in the mitochondria
what does DNP do?
DNP is lipid soluble, meaning it can cross into intermembrane space
allows protons to leak back into the matrix
DNP is a hydrophobic weak acid. The compound easily passes through the membrane of the mitochondria into the matrix. Once there, in the low H+ environment, it donates its proton. Since DNP is a lipid soluble molecule, it is allowed to cross into the inter-membrane space in both its protonated and deprotonated forms. Protons, which are normally pumped from the matrix to the inter-membrane space to facilitate ATP synthase, are picked up by DNP and moved back into the matrix, bypassing ATP synthase. Since these protons are leaking back into the matrix and the function of ATP synthase is disrupted, ATP can no longer be made here.
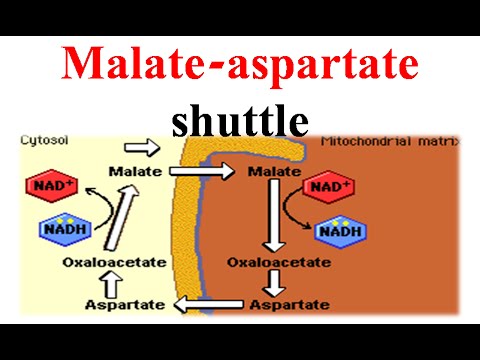
what is the malate-aspartate shuttle?
since NADH cannot cross into the mitochondrial membrane, this shuttle uses malate and aspartate as intermediates to move the reducing equivalents (electrons) into the mitochondrial matrix to generate ATP via OP
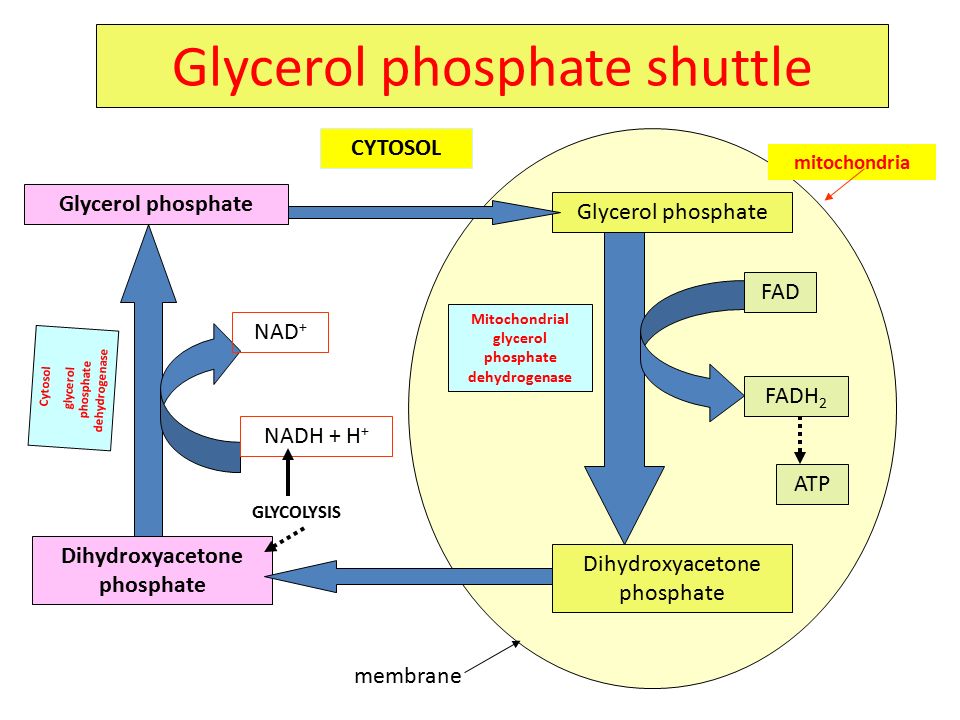
what is the glycerol-3-phosphate shuttle?
transfers electrons from cytoplasmic NADH into the mitochondria for ATP production since NADH can’t cross the inner mitochondrial membrane
converts DHAP to G3P via G3P dehydrogenase in a process that oxidizes NADH to NAD+
the mitochondrial G3P dehydrogenase then transfers the electrons from G3P to FAD to make FADH2 which donates the electrons to the ETC at complex II
do the NADH and FADH made in TCA cycle need a shuttle?
no, because they are made in the matrix (NADH → complex 1, FADH2 → complex 2)
why would someone on DNP have a higher temperature?
with low amounts of ATP being produced with this disruption, the cell's low energy charge signals the need for more ATP to be made, however, this process is now inhibited. metabolism is increased as a result and the energy that would have been used to make ATP has nowhere to go and instead is released as heat
The energy released from the electron transport chain is not all being harvested to catalyze ADP + P to ATP, and so it has to go somewhere. There is potential energy contained in the proton gradient. Every time a proton is moved down the concentration gradient, that is energetically favorable—release of free energy. Instead of this energy being used to make ATP through ATP Synthase, this energy is released as heat when a proton is transported across by DNP
why would someone on DNP have a higher respiration rate?
ATP production is decreased due to DNP disrupting the proton gradient → the energy charge of the cell would be low, signaling the cell that more ATP is needed in the cell
since ATP production is impaired, the cell detects this energy deficit and speeds up metabolism to compensate for this. however, with ATP production inhibited, this exergonic energy that would have been used to produce ATP has nowhere to go, leading to it being released from the body as heat
this increase in metabolism would cause the speed of the ETC to increase, meaning that electron carriers such as FADH2 and NADH would donate electrons at a faster rate and lead to an increase in the citric acid cycle to generate more of these electron carriers
this would increase CO2 as a byproduct
furthermore, more oxygen would be consumed in the process since oxygen acts as the ETC's final electron acceptor, increasing the patient's respiration rate to meet these needs.
Since less ATP is being made, the body responds by ramping up catabolic pathways to respond to the ATP depletion. Increased flux through the PDH complex and the citric acid cycle results in an increased production of carbon dioxide as well as increased production of NADH and FADH2 which give up their electrons to the electron transport chain. Oxygen is the final acceptor in the electron transport chain. Since DNP has no direct effect on the complexes of the electron transport chain, the body continues to use oxygen. Thus the patient experienced high respiration.
why would someone on DNP have widespread muscle rigidity?
since ATP production is inhibited, this energy cannot be utilized in the body for processes such as movement, which requires energy. low ATP levels prevent muscle relaxation which requires energy, leading to sustained contractions and rigidity in the body. furthermore, muscle contraction is controlled by calcium, of which requires ATP in order to be pumped out of the cytoplasm. without ATP, this active transport cannot occur, leaving calcium in the cytoplasm and muscle contraction to be sustained.
why is DNP effective?
dNP helps to increase the metabolism in the body due to the uncoupling of oxidative phosphorylation
as protons are allowed to pass ATP synthase, ATP cannot be produced here and an energy deficit in the cell will cause metabolism to increase to compensate, increasing processes such as the electron transport chain and the citric acid cycle
energy in the body that would have been used to make ATP is instead expended as heat
as the body strains for usable energy, this depletion causes an imbalance of energy and pushes the body to look for alternative energy sources, such as fat storage
as this fat is burned to use for energy, the patient loses weight as a result
what is leigh syndrome?
the body does not have a functional ATP synthase
how many molecules of ATP can be produced from 1 glucose in someone with leigh syndrome?
4 (SLP only)
ATP from substrate level phosphorylation in glycolysis: net of 2
ATP from substrate level phosphorylation in citric acid cycle: 2
No ATP could be made from NADH or FADH2. So the total possible is for from substrate level phosphorylation, compared to the normal 30-32 ATPs/glucose.
If ATP synthase is completely non-functional, the ETC will shut down because the gradient gets too steep. This means that NAD+ and FAD cannot be regenerated for the TCA cycle, which would then shut down, meaning no substrate-level phosphorylation in the TCA would occur. Glycolysis can still occur because pyruvate is reduced to lactate to oxidize NADH to NAD+ for glycolysis, without the need for the ETC.
what is brown fat?
a natural uncoupler of the ETC
generates heat in hibernating animals, is regulated so that its not expressed during summer