C3 - Chemical reactions
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Recall Avogadro’s constant
6.02×10^23
What is the formula to find moles
Moles = Mass/Mr
Explain observed changes in mass in non-closed systems
If a substance gains or loses mass, it is likely that a gas was involved in the reaction. This will not appear in the mass measurement and so the reaction may not appear to follow the law of conservation of mass.
Deduce the stoichiometry of an equation from the masses of reactants and products and explain the effect of a limiting quantity of a reactant.
The effect of a limiting quantity of reactant can cause the reaction to end before a desired amount of product is formed.
Masses can be calculated using the atomic mass multiplied by the amount of all of the atoms in the molecule
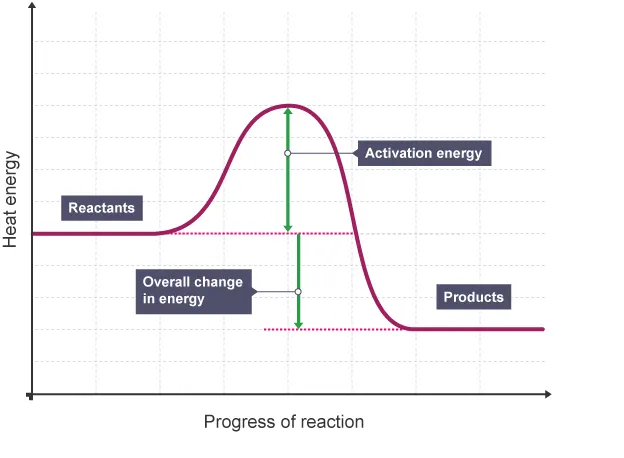
Draw and label a reaction profile for an exothermic reaction.
Draw and label a reaction profile for an endothermic reaction.
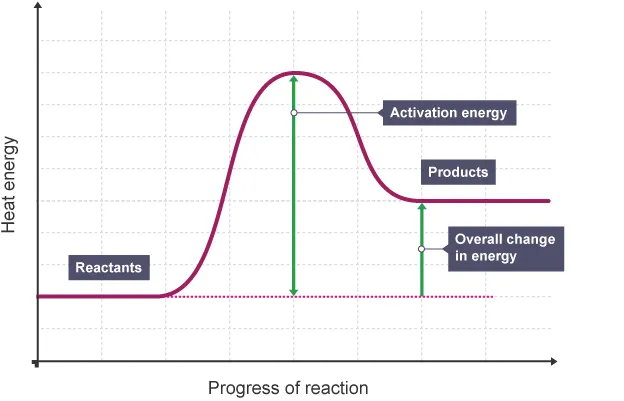
What is activation energy?
The energy required for a reaction to occur.
Identify oxidation and reduction, and identify their respective agents.
Oxidation is the loss of electrons, caused by an oxidising agent.
Reduction is the gain of electrons, caused by a reducing agent.
The substance that is oxidised is the reducing agent, and the substance that is reduce is the oxidising agent.
Recall the useful mnemonic
OILRIG
Oxidation
Is
Loss
Reduction
Is
Gain
What ions do acids and alkalis form?
Acids - Hydrogen ions - H+
Alkalis - Hydroxide ions - OH-
What is neutralisation.
Neutralisation is when an acid reacts with an alkali or a base to form a salt and water.
Define weak acids, and strong acids.
Strong acids are acids that ionise almost completely in water and usually have a very low ph. Almost all of the molecules ionise to form H+ ions.
Weak acids only partially ionise when in contact with water and usually have a ph of around 2-6. Only a small proportion of the molecules ionise to form H+ ions.
Explain Dilute and concentrated acids, and their differences to strong and weak acids.
Dilute acids have a low acid to solution ratio, meaning there is a lot more solute than acid.
Concentrated acids have a high acid to solution ratio.
You can have a dilute strong acid, and a concentrated weak acid.
Concentration of the acid has no affect on the pH or acidity of the acid.
Describe neutrality and relative acidity and alkalinity in terms of the effect of the concentration of hydrogen ions on the numerical value of pH.
If the pH of a solution decreases by one, the concentration of H+ ions increases by a factor of 10.
If the pH of a solution increases by one, the concentration of H+ ions decreases by a factor of 10.
This is additive so if the pH increases by 2, the concentration of H+ ions decreases by a factor of 100.
Describe a technique used to measure pH.
An indicator is a dye that changes colour depending on whether its above or below a certain pH. Universal indicator is a combination of dyes and is very useful for estimating the pH of a solution.
Describe an apparatus used to measure pH.
A pH probe attached to a pH can be used to measure pH electronically. The probe is placed in the solution you are measuring and the pH is given on a digital display as a numerical value. This gives a much more accurate reading as long as the probe is calibrated correctly and rinse it in between uses.
Describe the technique used to perform electrolysis.
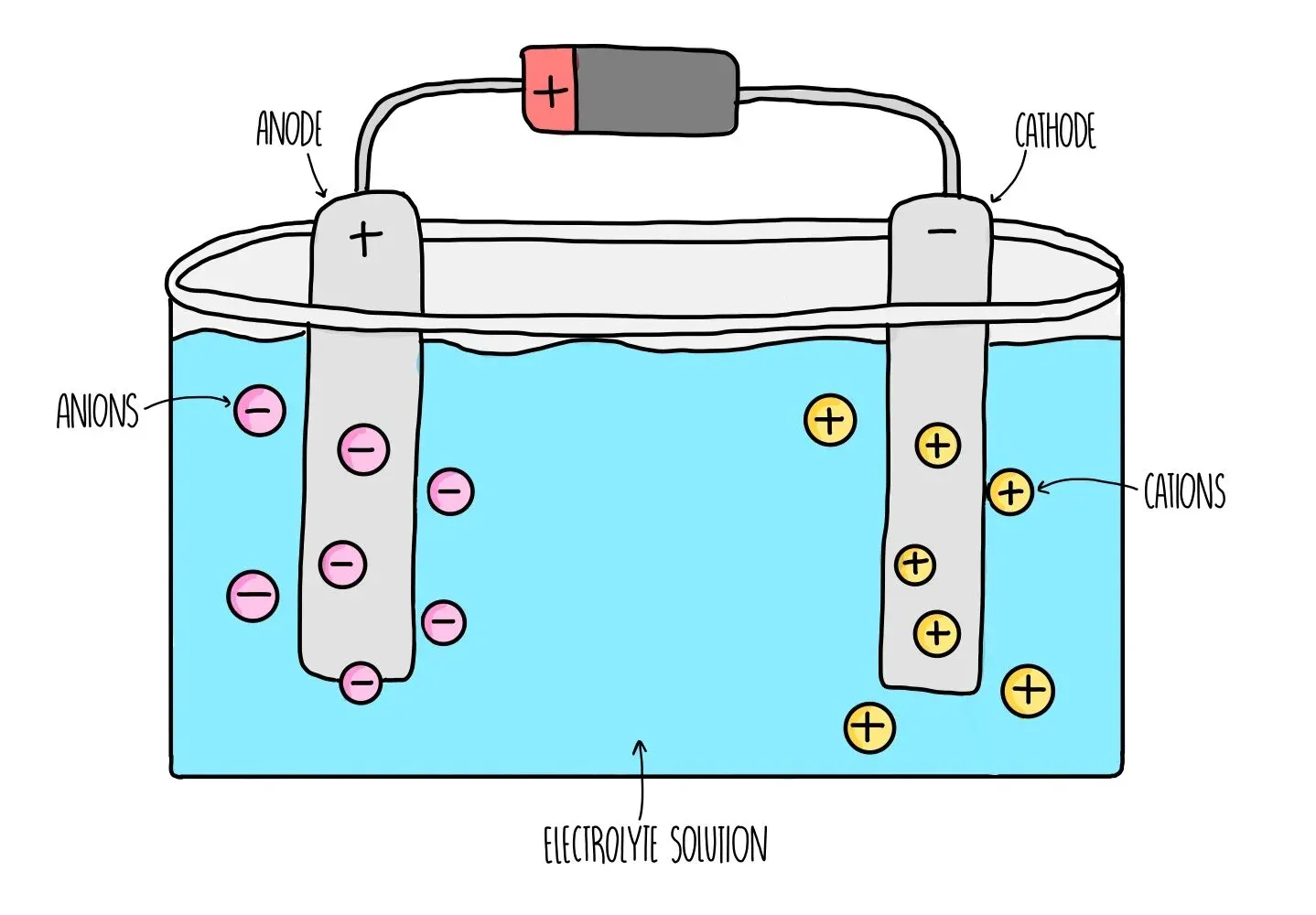
In electrolysis, what is formed at the cathode in a molten solution.
This is because of the fact that metals form positive ions, so are attracted to the cathode since the cathode is negatively charged, and gives the extra electrons to the metal.
In electrolysis, what is formed at the cathode in an aqueous solution?
If the metal is less reactive than hydrogen, the metal is formed at the cathode. This is because they have a greater tendency to gain electrons.
If the metal is more reactive than hydrogen, then hydrogen is formed at the cathode. This is because hydrogen will have a greater tendency to gain electrons.
In electrolysis, what is formed at the anode in a molten solution?
In a molten solution, non metals will be formed at the anode.
In electrolysis, what is formed at the anode in an aqueous solution?
If halide ions are present, then they will be formed at the anode as they have a greater tendency to lose electrons, as they are more reactive than oxygen.
If halide ions are not present, then oxygen will be formed at the anode as oxygen will have a greater tendency to lose electrons.
Describe the electrolysis of sodium chloride, with half equations for each electrode.
Use an aqueous solution of sodium chloride as the electrolyte.
Hydrogen will be formed at the cathode as sodium is more reactive, so hydrogen has a greater tendency to gain electrons.
2H+ + 2e- —> H2
Chlorine will be formed at the anode as it is more reactive than oxygen, meaning it has a greater tendency to lose electrons.
2Cl- —> Cl2 + 2e-
Describe the electrolysis of copper sulfate, and the half equations at the electrodes.
Aqueous solution of copper sulfate used as the electrolyte.
Copper is less reactive than hydrogen so copper is formed at the cathode.
Cu2+ +2e- —> Cu
No halide ions are present so oxygen and water is formed at the anode.
4OH- —> O2 + 2H2O + 4e-
Describe how you would use electroplating to coat an object. Use copper as an example.
Instead of using inert electrodes, a copper electrode can be used to electroplate an object. The object must be able to conduct electricity. Use a copper electrode at the anode, and place the object at the cathode and complete the circuit with a electrolyte of copper(II) sulfate. Run the electrolysis and stop when the object has been coated.