Chapter 5 - Electron configurations of chemical elements
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Spin states
Occurs when an electron is placed in a magnetic field, the electron generates a magnetic moment with two possible orientations.
This causes an electron to have two different spin states, thus producing the two oppositely directed magnetic moments.
This is also considered to be a quantum number.
Spin quantum number
Refers to the different spin states
It can only have the values +1/2 or -1/2
Pauli exclusion principle
“In a given atom, not two electrons can have the same four quantum numbers”
Since electrons in the same orbital have the same values of n, l and ml this postulate says that they must have different values of ms
Since there are only two allowed values of ms, each orbital can only hold two electrons, and they must have opposite spins.
How to calculate the energy levels in multi-electron atoms.
Where Zeff is the effective nuclear charge. Which is smaller than the real charge of the nucleus Z.
n is the quantum number.
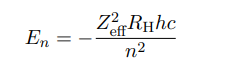
What is Zeff
Effective nuclear charge
Depends on both n and l
Zeff decreases for electrons further away (big n)
Electron has higher En
Shielding
For multi-electron atoms, each electron not only feels the attractive positive charge of the nucleus, but it also feels the repulsive negative charges of all the other electrons.
This increases the energy of the orbital.
When an electron is added to an ion, the attraction to the nucleus is weakened. The other electrons are shielding the outer electron from the full nuclear charge.
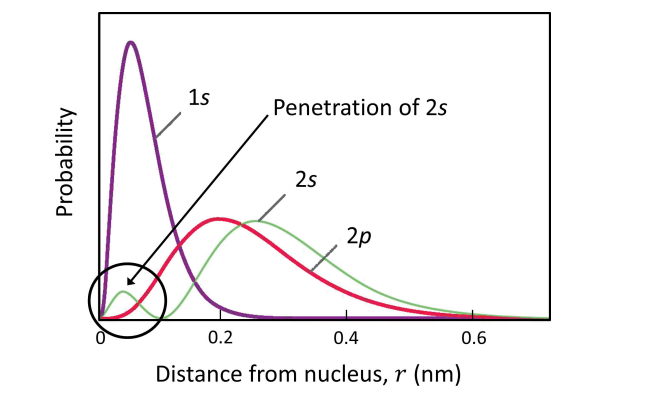
Penetration
If another electron penetrates the electron cloud than it comes closer to the nucleus
The shielding effect is reduced and the electron experiences the nuclear charge more fully.
The penetration of an electron depends on the shape of the orbital.
s>p>d>f, s is most likely to be penetrated.
Hund’s rule
When orbitals of equal energy are available, the electron configuration of lowest energy is the one with maximum number of unpaired electrons with parallel spins.
This is because electrons in different p orbitals are farther away from each other and therefore experience less repulsion than electrons in the same orbital.
Trends in ionization energy
The energy required to remove one electron from a gaseous atom in its ground state.
Ionization energy increases from left to right across a period because of increasing nuclear charge by the added protons.
This increased charge is only partially shielded by the electrons which are in the same n shell, so that Zeff increases across a period.
Going down a column the ionization energy decreases, because outer electrons are farther from the nucleus as n increases.
Elements with low ionization energy are strong reducing agents (they are oxidized themselves, which is loss of electrons)
What causes exceptions in ionization energy
E.g. from Be to B a new sub shell has to be added, so that creates a dip in ionization energy because now there is more shielding.
E.g. from N to O now in the 2p shell instead of there just being one electron in each there is another, so therefore there is electron pairing energy. Therefore there is a dip in ionization energy because the electron doesn’t mind being removed.
Trends in electron affinity
The energy that is released when an additional electron is added to a gaseous atom in its ground state.
Most elements have a positive affinity, because the extra electron is attracted by the positive nuclear charge.
Increases from left to right and from bottom to top. But is irregular.
This is because electron affinity depends not only on the effective nuclear charge, but also on whether there is a vacancy in a suitable orbital.
They are good oxidizing agents (reduce themselves, gain an electron)
Properties metals
Low ionization energy and low electron affinities, give up electrons easily and form positive ions.
Non-metals properties
High ionization energy and electron affinity.
Form negative ions
What is the only thing that determines orbital energy (in atoms with one electron)?
Only n
What do you call orbitals with the same energy?
Orbitals with the same energy are called degenerate
only occurs in atoms with one electron