Chemical Reactions of Aldehydes and Ketones
1/18
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
Nucleophilic Addition Reaction
Addition of Hydrogen Cyanide (HCN)
Aldehydes and ketones on reacting with HCN give cyanohydrins
Very slow reaction with pure HCN
So, it is catalyzed by a base and the generated CN- being a stronger nucleophile readily attacks on carbonyl carbon to yield corresponding cyanohydrin
Cyanohydrins are a useful synthetic intermediate
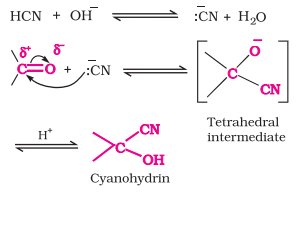
Nucleophilic Addition Reaction
Addition of Sodium Hydrogen Sulphite (NaHSO3)
Most aldehydes and aliphatic methyl ketones which are not sterically hindered on reaction with NaHSO3 give bisulphite addition product
Position of equilibrium lies largely to the right for most aldehydes and left for ketones due to steric reasons
Bisulphite addition compound is water soluble and can be converted back to the original carbonyl compound by treating it with dilute mineral acid or alkali
Useful for separation and purification of aldehydes
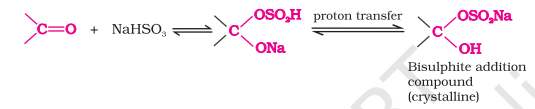
Nucleophilic Addition Reaction
Addition of Grignard’s Reagent
Aldehydes and ketones on reaction with Grignard’s reagent give addition products which upon hydrolysis with water or dilute mineral acids give alcohols
The type of alcohol depends upon the aldehyde/ketone used
Formaldehyde → 1* alcohols
Other aldehydes - 2* alcohols
Ketone → 3* alcohols
Nucleophilic Addition Reaction
Addition of Alcohols
Aldehydes react with one equivalent of monohydric alcohol in the presence of dry HCl gas to yield alkoxy alcohol intermediates known as hemiacetals
Hemiacetals on further reaction with one more molecule of alcohol give acetals
Ketones do not react with monohydric alcohols but they react with dihydric alcohol such as ethylene glycol to give cyclic ketals
Acetals and ketals hydrolysed by dilute acids generate original aldehydes and ketones
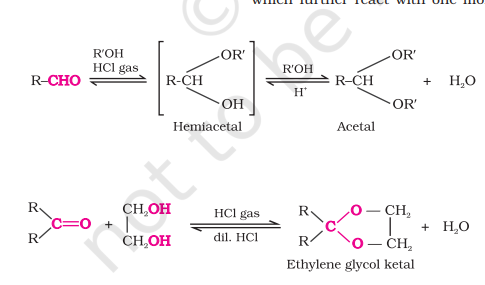
Nucleophilic Addition Elimination Reaction
Addition of Ammonia and its Derivatives
Aldehydes and ketones react with ammonia and many derivatives (H2N-Z) in weakly acidic mediums to form compounds containing >C=N group
Reactions are reversible and catalysed by acids
Equilibrium favors product formation due to rapid dehydration of the intermediated to form >C=N-Z type compounds
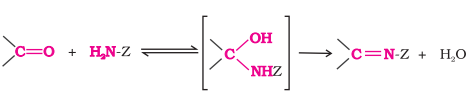
Quick Note
Brady’s Reagent
2,4-dinitrophenol hydrazine
Aldehydes and ketones react to form yellow, orange or red precipitate of 2,4-dinitrophenyl hydrazone
Useful for characterization of aldehydes and ketones
Oxidation
Difference in Oxidation of Aldehyde and Ketone
Aldehydes can be oxidized to carboxylic acids easily in the presence of both weak and strong oxidizing agents due to the presence of H-atom on the carbonyl group. This helps to easily convert into -OH group without any cleavage. The carboxylic acid contains the same number of carbons as the parent aldehyde.
Ketones do not contain the H-atom on carbonyl group, therefore can only be oxidized via strong oxidizing agents like KMnO4, HNO3 etc. It involves cleavage of carbon-carbon bond therefore reducing the number of carbons in the carboxylic acid compared to parent ketone.
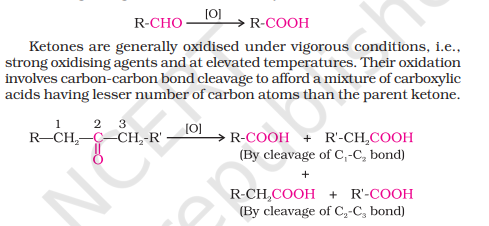
Oxidation
Tollen’s Test
Known as silver mirror test ; aliphatic and aromatic aldehydes reduce Tollen’s reagent
Aldehyde heated with freshly prepared Tollen’s reagent (ammoniacal solution of silver nitrate) a silver mirror is formed due to formation of silver metal
Aldehyde oxidizes to corresponding carboxylate anion
Reaction occurs in alkaline medium

Oxidation
Fehling’s Test
Fehling’s Solution A - aqueous copper sulphate solution
Fehling’s Solution B - alkaline sodium potassium tartarate (Rochelle’s salt)
These solutions are mixed in equal proportion before test
When aldehyde is heated with Fehling’s reagent, reddish brown precipitate is formed. Aldehyde oxidizes to corresponding carboxylate anion
Aromatic aldehydes do not reduce Fehling’s solution

Oxidation
Benedict’s Test
Benedict’s reagent : Cu2+ ions that are complexed with citrate ions (unlike Fehling’s)
Reacts same way as Fehling’s
Oxidation
Oxidation of Methyl Ketones by Haloform Reaction
Aldehydes and ketones having one methyl group attached to the carbonyl carbon (methyl ketones) are oxidized by sodium hypohalite to corresponding sodium salts of carboxylic acids
Methyl group gets converted to haloform
Carbon-carbon double bond remains unaffected by this reaction, if present
Iodoform reaction is used to detect CH3CO- group/CH3CH(OH)- group that oxidizes to CH3CO-
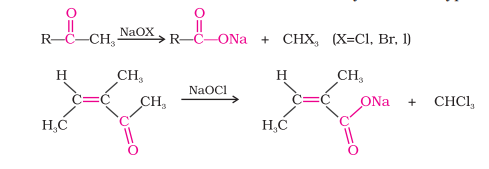
Reduction
Reduction to Hydrocarbons (Clemmensen Reduction)
Reducing the carbonyl group to a -CH2 group
Carried out in the presence of Zn amalgam and conc. HCl
Generally used for aldehydes and ketones that are sensitive to alkalis
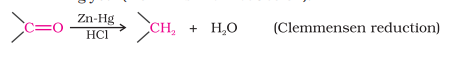
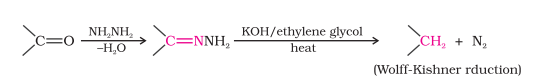
Reduction
Reduction to Hydrocarbons (Wolff-Kishner Reduction)
Aldehydes and ketones are heated with hydrazine and NaOH/KOH in high boiling solvent like ethylene glycol
Useful for carbonyl compounds that are sensitive to acids
Reduction
Reduction with HI and Red P
Aldehydes and ketones on reacting with hydroiodic acid (HI) and red phosphorus at 423K get reduced to their corresponding alkanes
Reduction
Reduction to Alcohols
Aldehydes and ketones can be reduced to 1* and 2* alcohols respectively either by catalytic dehydrogenation or chemically via LiAlH4/NaBH4
Reduction due to α-Hydrogen
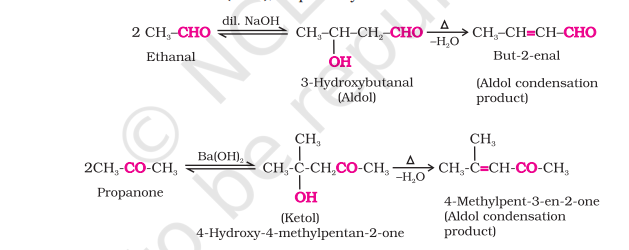
Aldol Condensation
Aldehydes/ketones with α-H atom undergo reduction in the presence of dilute alkali as catalyst to form aldols/ketols
Name comes from the two functional groups : aldehyde/ketone + alcohol
Aldols readily lose water to α, β unsaturated carbonyl compounds (aldol condensation products)
Formaldehyde, benzaldehyde, benzophenone do not undergo aldol condensation due to absence of α-H
Reduction due to α-Hydrogen
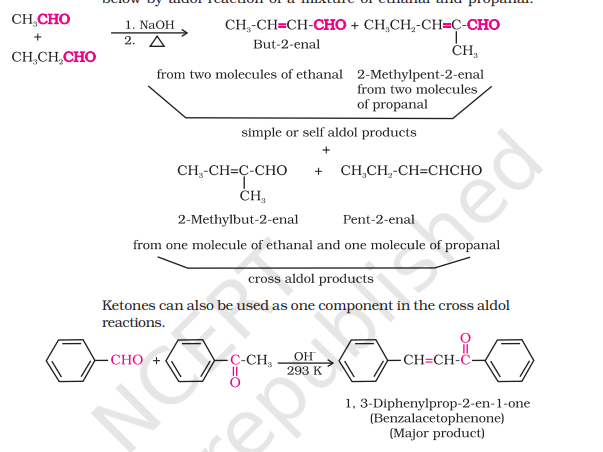
Cross-Aldol Condensation
When aldol condensation comprises of two different aldehydes/ketones
Both reactants having α-H atom will result in a mixture of 4 products due to cross condensation and self condensation
Great synthetic use even if one of the compounds is not having α-H atoms
Ketones can also be one component
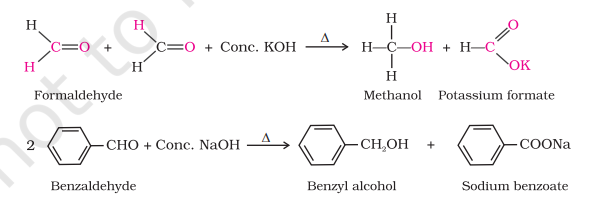
Cannizzaro Reaction
Aldehydes that do not have α-H atoms undergo self oxidation and reduction disproportionately on heating with conc. alkali
One molecule reduces to alcohol, other oxidizes as salt of carboxylic acid
Electrophilic Substitution Reaction
Aromatic aldehydes and ketones undergo electrophilic substitution reactions like halogenation, sulphonation, nitration
Due to electron withdrawing nature, deactivating and meta-directing