ch. 16 - amino acids, proteins, enzymes
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
89 Terms
what is the most abundant type of molecule present in our bodies
proteins
which food are proteins found in
eggs, nuts, dairy, etc.
what are the classes of proteins
structural, contractile, transport, storage, hormone, enzyme, and protection
what do structural proteins do
provide structure to our bodies
what do contractile protein do
muscle movement
transport protein function
move nutrients thorugh the body
storage protein function
store nutrients
hormone protein function
regulate metabolism and nervous system
enzyme protein function
catalyze (speed up) reactions
protection protein function
destroy foreign substances
what are amino acids
the building blocks of proteins (monosaccharides)
what are the components of the amino acid backbone
carboxylic acid, amine function groups, and an R group
at our body’s pH, _____’s ends are both ionized
amino acid
which functional groups are characterized as nonpolar when considering nonpolar amino acids
alkanes and benzenes
can amino acids be slightly polar and still be considered nonpolar?
yes
which functional group is characterized as polar acidic when discussing polar amino acids
carboxylic acid
which functional groups are characterized as neutral when discussing polar neutral amino acids
alcohol, thiol, amide
which functional group is characterized as polar basic when discussing polar basic amino acids
amine
what are peptide bonds
when two amino acids link together
what are two connected amino acids called
dipeptide
what are three connected amino acids called
tripeptide
what are four connected amino acids called
tetrapeptide
what is the rule when naming peptide bonds
only keep the original name of the last amino acid, the rest before the last have to change their last syllable to -yl
which two factors give proteins their biological activity
shape and structure
what four categories determine the shape of a protein
primary structure, secondary structure, tertiary structure, quaternary structure
what does the primary structure entail
determined by the order of amino acids in the protein
moiety definition
an individual amino acid in a peptide or protein
proteins can contain tens to tens of thousands of amino acid ____ in many different orders
moieties
how does the secondary structure form
when the backbones of amino acids interact with each other via hydrogen bonds
what are the three most common secondary structures for proteins
alpha helix, beta-pleated sheet, and triple helix
how is tertiary structure determined
by how the protein folds
hydrogen bonds in tertiary structure R groups
interactions between OH, NH, and water (two polar)
salt bridges in tertiary structure R groups
interactions between ionized R groups (two ionic)
disulfide bonds in tertiary structure R groups
S-S bonds (two sulfur)
hydrophobic interactions in tertiary structure R groups
interactions of nonpolar amino acid R groups (two nonpolar)
what does the quaternary structure of proteins entail
how different protein units attach to one another
how do biologically active proteins form
when two or more tertiary structures attach to one another
the subunits of quaternary structures are held together by which bonds
hydrogen bonds, disulfide bonds, salt bridges, etc.
____ is made of four separate tertiary structures held together into one protein
hemoglobin
how do proteins get their structure?
DNA contains a series of instructions on what amino acids need to be strung toghether and when to allow the protein to fold
origin of DNA
nucleus of every cell
what happens when proteins are denatured
secondary, tertiary, or quaternary structure of the protein is disrupted
how can protein denaturation be caused
heat, acids or bases, organic compounds, heavy metals, and agitation
what temperature denatures proteins
over 50 degrees celsius
how do acids and bases denature proteins
disrupt salt bridges and hydrogen bonds
how do organic compounds denature proteins
disrupt hydrophobic interactions
how do heavy metals denature proteins
break disulfide bonds
how does agitation denature proteins
stretches peptide bonds and breaks hydrogen bonds and hydrophobic interactions
are most denaturation reversible or irreversible
irreversible
what are enzymes
catalysts made of protein
what do enzymes do
speed up chemical reactions in the body by lowering the activation energy of the reaction
how often does enzymatic activity occur outside the body
once every 27 seconds
when one molecule of the enzyme carbonic anhydrase is present for a reaction in our body, how often will the reaction occur
1 million times per second
list all the types of enzymes
oxidoreductase, transferase, hydrolase, lyase, isomerase, ligase
what does oxidoreductase do
catalyze redox reactions
what does transferase do
transfer substituents from one molecule to another
what does hydrolase do
hydrolyze (break apart) molecules using water molecules
what does lyase do
add or remove groups without using water
what does isomerase do
rearrange atoms in a molecule
what does ligase do
combine molecules together using energy
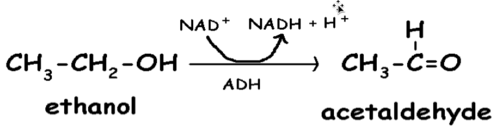
what reaction does this figure display
oxidoreductase
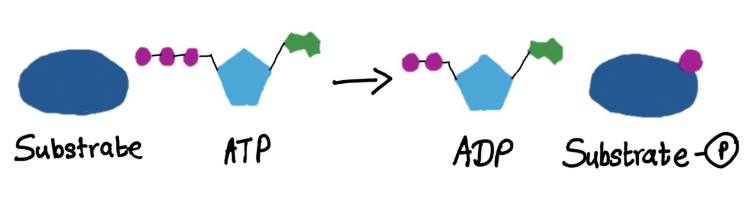
what reaction does this figure display
transferase
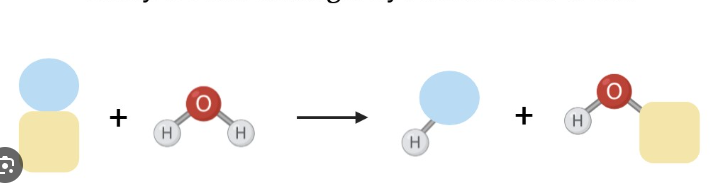
what reaction does this figure display
hydrolase
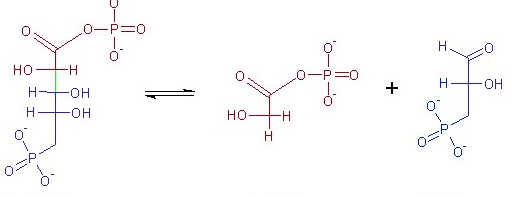
what reaction does this figure display
lyase
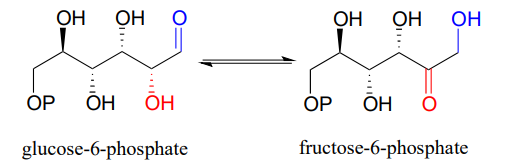
what reaction does this figure display
isomerase
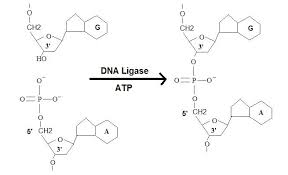
what reaction does this figure display
ligase
substrate definition
the molecule(s) that react in the enzyme
active site definition
the location in the enzyme molecule where the reaction takes place
enzyme-substrate complex definition
the combination of the enzyme and substrate before the reaction takes place
cofactor definition
metal ions or vitamin derivatives that make an enzyme function
how do enzymes work 1st step
enzyme has to bind to the substrate
how do enzymes work 2nd step
once bound, reaction can happen or they can split apart without a reaction
factors affecting enzymatic activity
temperature, pH, and inhibitors
temperature optimal for enzymatic activity
37 degrees celsius
what happens to enzymes at colder temperatures
they work slower
what happens to enzymes at higher temperatures
they become denatured
which conditions in the body would cause significant temperature change that would hinder enzymatic activity
hypothermia and high fever
what is the optimal pH for enzymes to work
depends on where enzyme is active in the body
what happens if the pH is too high or low for enzymes
enzyme is denatured
what is an inhibitor
a molecule that prevents an enzyme from reacting
what are the four different types of inhibitors
competitive reversible, competitive irreversible, noncompetitive reversible, and noncompetitive irreversible
what occurs in competitive inhibition
inhibitor has a similar shape to substrate and will fit into the active site without reacting
how to restore activity from a competitive inhibitor
concentration of substrate must be increased
what occurs in noncompetitive inhibition
bind to another part of the enzyme and cause active site to change in shape to no longer recognize the substrate
how do you regain activity from a noncompetitive inhibitor
inhibitor must be chemically removed
what occurs in an irreversible inhibition
inhibitor forms a covalent bond with amino acid in the active site or other place in the enzyme, bond cannot be broken by the body, so the enzyme is permanently inhibited
what is an example of an irreversible inhibition
antibiotics
what is an example of a competitive inhibitor
penicillin
what is penicillin
a competitive inhibitor for an enzyme in bacteria that makes a compound necessary for bacteria to expand their cell walls