Antibacterials & Antifungals & emerging therapeutics
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
What are the 3 types of antibacterials?
1. Small molecule drugs E.g. Antibiotics
2. Antibacterial peptides 3. Bacteriophage
Note: This lecture focuses on 2 and 3
What are bacteriophages?
viruses that infect bacteria
Recap: What are the 4 levels of structural organisation for proteins and briefly explain them
Primary structure:
-linear sequence of amino acids in a polypeptide chain, held together by peptide bonds
-Determined by genetic code
Secondary Structure:
-local folding of the polypeptide chain into specific structures due to hydrogen bonding between the backbone atoms
-e.g. alpha helix and beta sheet
Tertiary structure:
-overall 3D shape of a single polypeptide chain
-stabilised by interactions between the side chains (R groups) of amino acids, including:
Hydrogen bonds
Ionic bonds
Disulfide bridges (covalent bonds between cysteine residues)
Hydrophobic interactions
Quaternary structure:
-proteins composed of two or more polypeptide chains (subunits) that function together as a unit
-Held together by same interactions as tertiary structure
-e.g. haemoglobin has four polypeptide chains (two alpha and two beta subunits).
What are the classifications for antibacterial peptides?
-alpha helix
-extended or non alpha/beta
-alpha/beta mixed or Beta-hairpin or loop
-Beta sheet
What are the 2 sources of antimicrobial peptides?
Can be natural or synthetic
What is the broad purpose of antibacterial peptides?
natural defense molecules in the immune system, playing a crucial role in host protection against infections
What are the 5 ways they carry out their function?
1) Direct Killing of Microorganisms
2) Immune System Modulation (response to infections)
3) Prevention of Antibiotic Resistance
4) Wound Healing and Tissue Repair
5) Neutralisation of Bacterial Toxins
What are the 3 mechanisms of action of antibacterial peptides?
1) Cell membrane damage
2) Intercellular activity
3) Immune Regulation
How do antibacterial peptides cause cell membrane damage?
-Antibacterial peptides are typically cationic (positively charged) and amphipathic (have polar and non-polar regions), allowing them to interact with the negatively charged bacterial membranes and insert into the lipid bilayer
e.g.
-Barrel-stave model :
-Antibacterial peptides bind to bacterial membrane. Then insert into bilayer and form pore-forming-structures = barrel like channels = disrupts membrane integrity = leakage of ions and macromolecules= cell death
Why do antimicrobial peptides preferentially target microbes and not hum cells for example?
-Antibacterial peptides are typically cationic (positively charged) and amphipathic (have polar and non-polar regions), allowing them to interact with the negatively charged bacterial membranes and insert into the lipid bilayer. Human cells are neutral.
-Content of lipids are different
How do antibacterial peptides effect intercellular processes?
-They penetrate the cytoplasm and interfere with various metabolic pathways, leading to bacterial death
-Inhibition of DNA (bind to DNA), RNA, or Protein Synthesis (by interfering with ribosomal activity)
-Inhibition of Enzymatic Functions (disrupting energy production)
-Generation of Reactive Oxygen Species (ROS) = oxidative stress -> DNA fragmentation and cell death
How do antibacterial peptides effect immune regulation
• Reducing endotoxin-induced inflammatory response
• Inducing synthesis of pro-inflammatory factors
• Adjusting adaptive immunity,
• Inducing secretion of cytokines and subsequently recruiting macrophages to exert immune modulatory effects
What are three advantages of antimicrobial peptides (AMPs)?
1️⃣ Broad-Spectrum Activity:
-can kill Gram-positive and Gram-negative bacteria, fungi, viruses, and even parasites
-Rapid acting
2️⃣ Low Resistance Development:
Unlike traditional antibiotics that target specific bacterial pathways (e.g., protein synthesis), AMPs disrupt the bacterial membrane, which is harder for bacteria to modify without losing viability.
-Promising solution to multi-drug-resistant (MDR) bacteria
3️⃣ Immune Modulation & Wound Healing:
-They do more than just kill pathogens. They also modulate the immune response to prevent excessive inflammation
-Some AMPs promote wound healing by stimulating cell growth and reducing inflammation
What are three challenges and/or problems associated with AMPs?
1️⃣ Stability & Degradation in the Body:
-AMPs are easily broken down by proteases in the body, leading to short half-lives and reduced effectiveness
-Easily degraded in digestive system so hard to administer orally
2) Expensive and hard to produce
3️⃣ Potential Toxicity to Host Cells:
-Some AMPs can cause cytotoxicity or haemolysis (damage to red blood cells) at high concentrations
-Non-specific membrane disruption could lead to side effects
Name an example of emerging antimicrobial therapeutic and describe its mechanism of action?
Bacteriophage-Based Treatment (Phage Therapy)
Bacteriophage therapy is an emerging antimicrobial approach that uses viruses (bacteriophages) to specifically target and kill bacteria, offering a potential solution to antibiotic-resistant infections
Mechanism of Action:
Specific Binding: Phage attaches to bacterial surface receptors.
DNA Injection: Viral genetic material enters the bacterium.
Replication: Phage hijacks bacterial machinery to produce new viruses.
Lysis: Bacterial cell bursts, releasing new phages to infect others.
What are the characteristics of bacteriophages?
-High host specificity - usually one species
-2 mechanisms to infect bacteria: ytic and non-lytic phages
-Heat sensitive
-Have many shapes and sizes
-They are involved in transduction - when bacteriophages accidentally carry bacterial DNA from one bacterium to another during infection. The new bacterium may incorporate the foreign DNA into its own genome, gaining new traits (e.g., antibiotic resistance or toxin production) = evolution bacteria
-Can replicate via lytic and lysogenic
-Used in diagnosis and treatment
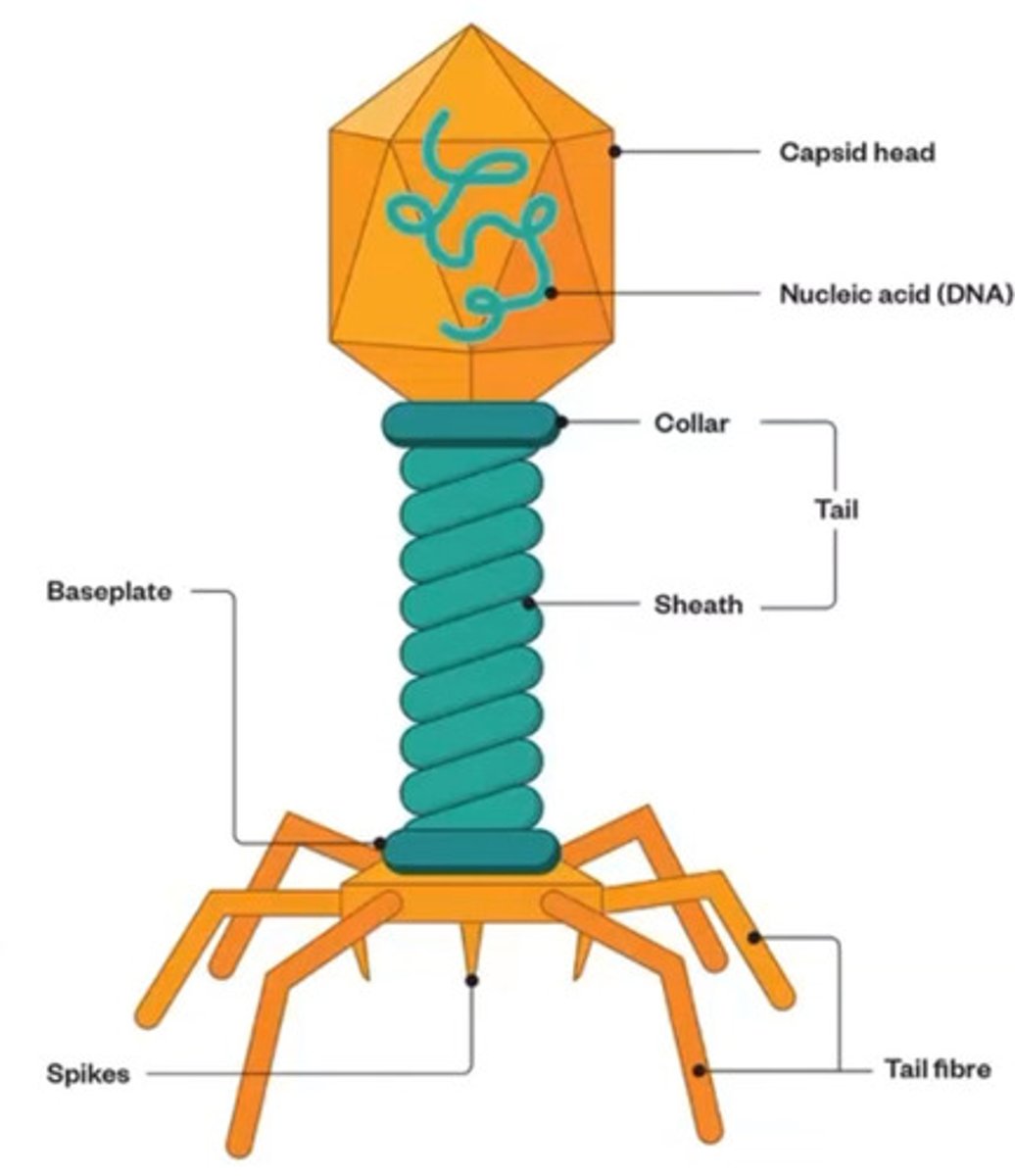
Bacteriophage structure and their functions?
hint: there are 5
• Most phages are tadpole shaped
• Capsid Head: Hexagonal in shape, composed of coat protein. It encapsulates phage DNA
• Genome DNA: double stranded DNA codes for enzymes and protein necessary to replicate more viruses
• Tail sheath: hollow core covered with contractile sheath. When infecting a bacteria DNA travels from head to bacteria through sheath
• Tail fiber: helps anchor the phage on the cell membrane
•Spike: a protein complex that penetrates into the host cell membrane during infection and facilitates injection of bacteriophage DNA into the host cells
How do bacteriophages replicate lytic way?
1) Adsorption
2. DNA entry
3. DNA replication (DNA is not integrated in host DNA)
4) Transcription and translation
5) Phageassembly
6) Host cell lysis
How do bacteriophages replicate lysogenic way?
1. Adsorption
2. Penetration
3. DNA integration (The phage DNA integrates into the host chromosome, forming a prophage)
4. DNA replication
5. Induction of daughter cell
6. Phage DNA and proteins are synthesised
7. Phage DNA assemble to form new virus
8. Release after lysis of host
Main difference between lysis and lysogenic mechanism of bacteriophage replication ?
Lysogenic involves DNA integration, Lysis does not
What is the clinical use of bacteriophages?
-Used as an antibacterial strategy especially due to rise in antibiotic resistance
-Phage therapy is done by isolating and identifying the pathogenic strain of bacteria a patient is infected with and carrying out in vitro screening of specific lytic phages. Safety and efficacy trials are carried out, formulations are tested and then administered.
What are some of the limitations of phage therapy?
1️⃣ Narrow Host Range - as they are highly specific
2️⃣ Potential for Bacterial Resistance - by modifying their surface receptors or using CRISPR-Cas immune systems
3️⃣ Regulatory and Safety Concerns
4️⃣ Short Half-Life in the Body - may cleared quickly by the immune system and trigger immune reactions also
5️⃣ Difficulties in Production and Storage - Phages need to be carefully isolated, purified, and stored to maintain stability.
-phage preparations must be customised for each infection, making large-scale production more complex
6️⃣ Limited Research and Clinical Trials
What are the 2 reproductive cycle of fungal infections?
-Sexual - involves genetic recombination = fungal diversity
-Asexual - clones
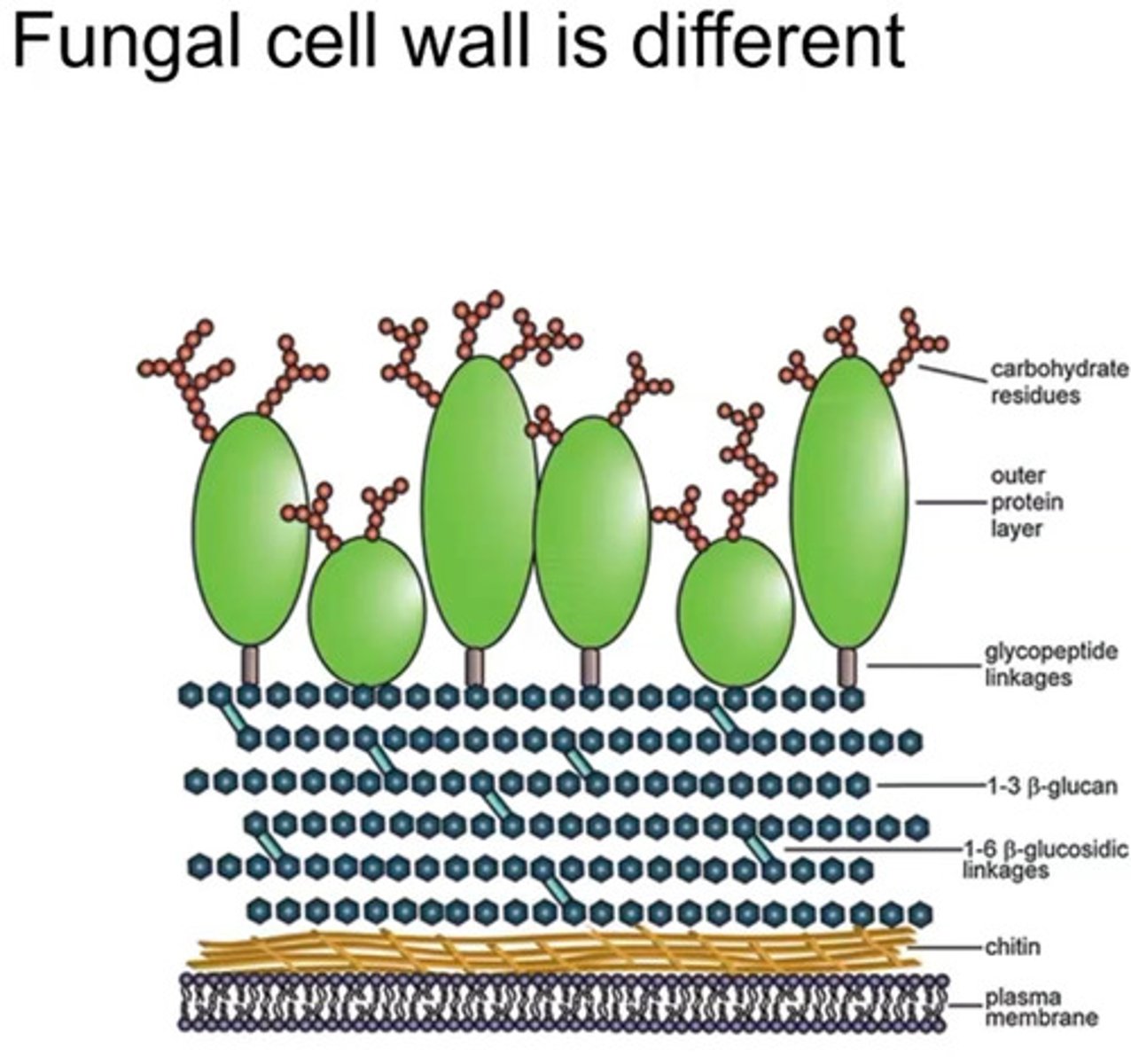
Structure of fungal cell wall
-FUNGAL CELL WALLS HAVE CHITIN, HUMAN AND BACTERIA CELLS DONT HAVE THIS
Patient comes to you and you don't know the source of infection between viral and bacteria - what test would you do to identify which one it is?
CHITIN TEST: used to detect the presence of fungal cell walls in clinical samples or environmental settings to diagnose fungal infections
Sample collection: blood, tissue biopsies, sputum etc.
Detection: test typically uses specific binding molecules or chemical reagents that can identify chitin or chitin-like molecules
Techniques used: fluorescent staining, microscopy, or enzyme-linked assays
How does the immune system recognise fungi as threats?
-Fungi have pathogen associated molecular patterns (PAMPS)
-The immune system has pattern recognition receptors (PRRs) that detect these signals and trigger a defense response.
What are the routes of invasion of fungal pathogens?
-Inhalation
-Skin wounds/damaged tissue
-Mouth
-Lungs
-Vagina
-Circulation
What is the purpose of morphological modulation (changing their shape and structure) of fungi cells?
avoid being destroyed by immune cells
Examples:
-Switching between yeast and filamentous forms (e.g., Candida).
-Hiding inside immune cells and preventing their destruction.
-Forming protective capsules that make them harder to attack.
How can fungi enter the systemic system?
1️⃣ Inhalation of spores/conidia
2️⃣ Entry into alveoli
3) Immune cells attempt to stop infect but if overwhelmed infection spread = pneumonia and lung nodules
4) Macrophages try to contain the infection by encapsulating fungal cells in small structures called granulomas.
5) Some fungi survive inside macrophages, escape, and enter the bloodstream, where they can spread to other organs, including the brain (via the blood-brain barrier).
What are the 5 classes of antifungal drugs?
azoles, polyenes, echinocandins, allylamines and pyrimidine analogues
MOA of Azoles e.g. fluconazole
MOA: Inhibit lanosterol 14α-demethylase, an enzyme involved in ergosterol synthesis (ergosterol is a key fungal cell membrane component).
Effect: Disrupts cell membrane integrity, increasing permeability and leading to fungal cell death.
MOA of Polyenes (e.g., Amphotericin B,)
MOA: Bind directly to ergosterol in the fungal cell membrane, creating pores.
Effect: Cell membrane leakage → ions and molecules leak out → cell death.
Side Note: Amphotericin B is highly toxic (nephrotoxicity common
MOA of Echinocandins
MOA: Inhibit β-1,3-glucan synthase, which is essential for fungal cell wall synthesis.
Effect: Weakens the fungal cell wall, leading to osmotic instability and lysis.
MOA of allylamines
MOA: Inhibit squalene epoxidase, an enzyme required for ergosterol synthesis.
Effect: Ergosterol depletion weakens the fungal cell membrane, and squalene accumulation becomes toxic to fungi
MOA of pyrimidine analogoues
MOA: Flucytosine is converted into 5-fluorouracil (5-FU) inside the fungal cell, which disrupts DNA and RNA synthesis.
Effect: Inhibits fungal protein production and cell division, leading to cell death.
Random: What is transcription?
the process of making an RNA copy of a gene's DNA sequence
• What are the common mechanism between antimicrobial and antifungal resistance?
• What are differences?
1️⃣ Enzymatic Drug Modification
Bacteria: Produce enzymes like β-lactamases to break down antibiotics (e.g., penicillins).
Fungi: Produce enzymes that modify antifungals (e.g., resistance to flucytosine through mutations in cytosine deaminase).
2️⃣Alteration of Drug Target
Bacteria: Modify targets (e.g., mutated penicillin-binding proteins in MRSA resist β-lactams).
Fungi: Modify lanosterol 14α-demethylase (target of azoles) to reduce drug binding.
3️⃣ Efflux Pumps (Pumping drugs out of the cell)
Bacteria: Use multidrug efflux pumps (e.g., AcrAB-TolC in Gram-negative bacteria).
Fungi: Overexpress ABC transporters (e.g., CDR1, CDR2) and MFS transporters (e.g., MDR1) to remove azoles.
4️⃣ Biofilm Formation (Protective shield against drugs)
Bacteria: Form biofilms on surfaces (e.g., Pseudomonas aeruginosa).
Fungi: Candida spp. biofilms reduce drug penetration and enhance resistance.
How do antifungal peptides effect intercellular processes?
-They penetrate the cytoplasm and interfere with various metabolic pathways, leading to bacterial death
-Inhibition of DNA (bind to DNA), RNA, or Protein Synthesis (by interfering with ribosomal activity)
-Inhibition of Enzymatic Functions (disrupting energy production)
-Generation of Reactive Oxygen Species (ROS) = oxidative stress -> DNA fragmentation and cell death
How do antifungal peptides effect the cell wall of fungi?
-Inhibition of cell adhesion (prevent fungal cells from attaching to surfaces or host tissues)
-Extracellular matrix (in fungal biofilms protects fungi from antifungal drugs) destabilisation = increases drug penetration - fungi easier to eliminate
-Intracellular mechanisms that trigger antibiofilm activities (= fungal stress responses, leading to cell wall weakening and apoptosis
What is the function of biofilms ?
Biofilms are communities of microorganisms that attach to surfaces and form a protective matrix and protects the microorganisms against the host immune system
What is mRNA
messenger RNA carries the DNA message from the nucleus to the ribosomes
What are 4 Common mechanism of RNA-based therapeutics and what are they- ask tutor
-Specific translation - provide synthetic mRNA that directs ribosomes to produce a specific therapeutic protein e.g. mRNA vaccine
-Post-transcriptional expression modulation:
Down regulation:
RNA interference (RNAi) prevents translation, reducing protein production
Upregulation:
Synthetic RNA or modified miRNA mimics can stabilise mRNA and increase protein production.
-Expression modulation/gene modification - CRISPR-Cas9 and RNA-guided gene editing use guide RNA (gRNA) to target specific DNA sequences for modification. mRNA encoding gene-editing enzymes can introduce permanent genetic changes
-Protein binding/inhibition - Some RNA molecules directly bind to proteins, blocking their function. (Aptamers are short RNA or DNA sequences that mimic small molecules and competitively inhibit protein activity.)
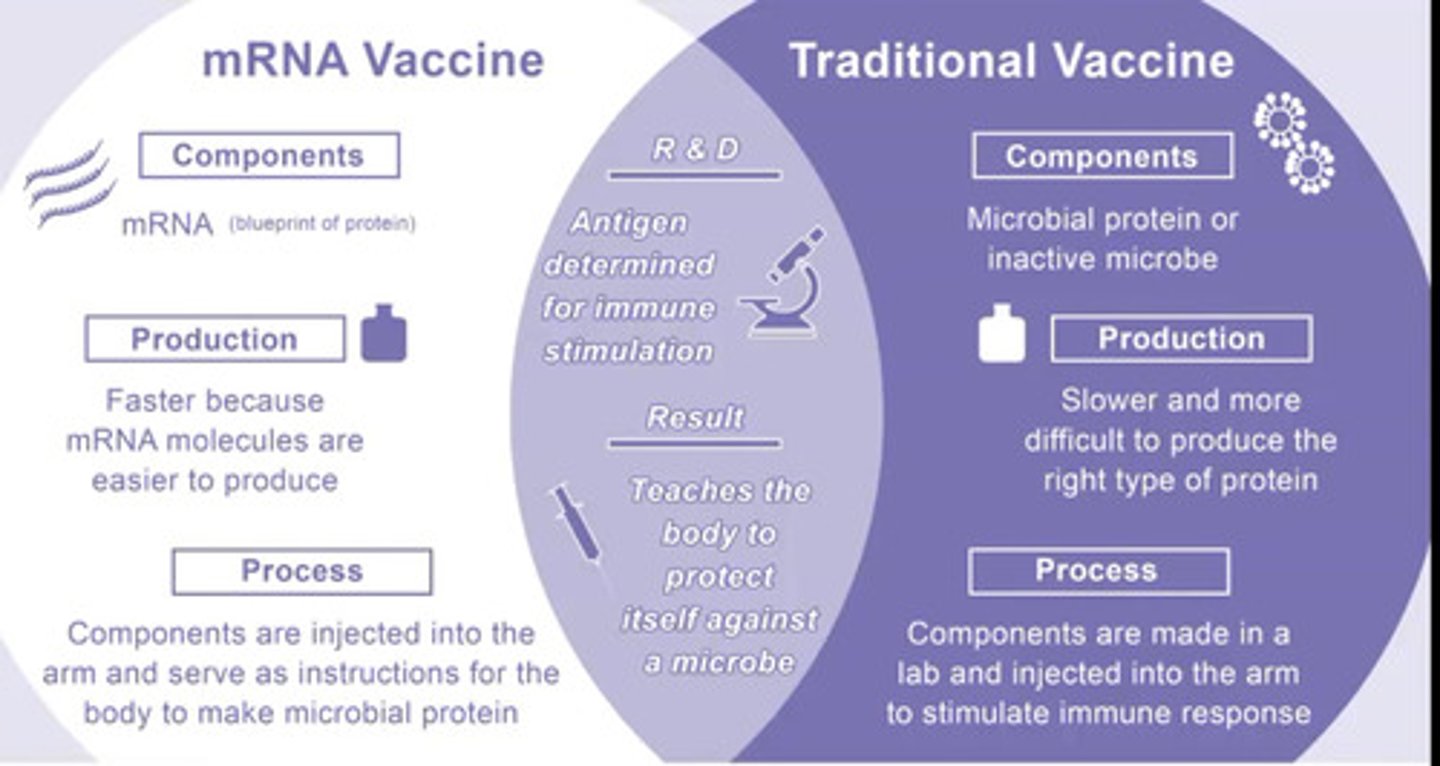
Why is mRNA vaccine more popular that traditional vaccine?
-Faster because mRNA molecules are easier to produce
-Cheaper
-Components are injected into the arm and serve as instructions for the body to make microbial protein
-Use of mRNA (protein blueprint)
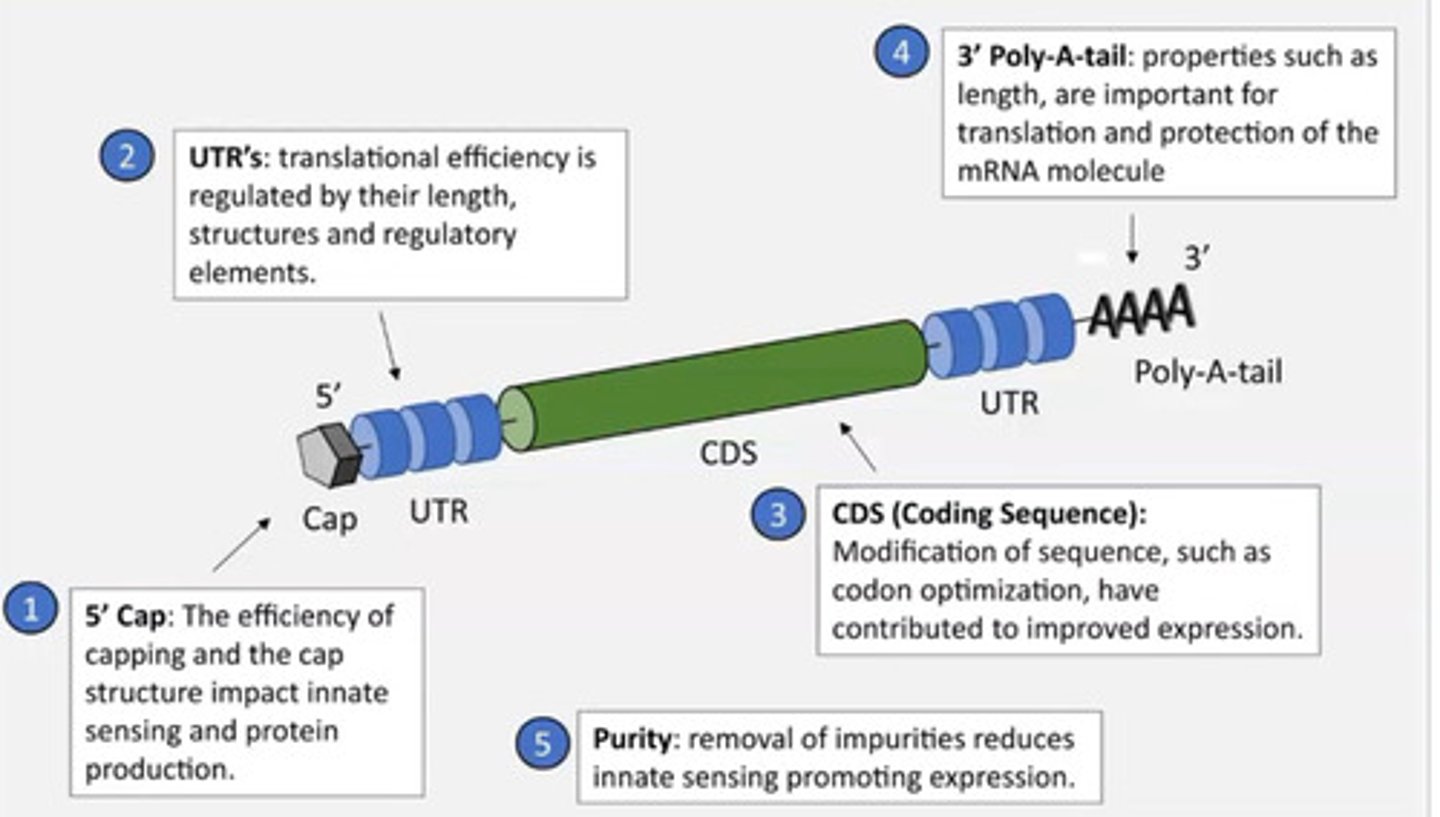
What is the mRNA vaccine structure and design? Why is it designed like this?
-matches the structure of mRNA in the body
-To avoid immune response
-If it didnt have a cap the body would recognise it as non-self and destroy it
-Also for translation
Note: the ribosome recognises the cap then begins translation
What is the MOA of mRNA vaccines?
1) mRNA Delivery - vaccine contains synthetic mRNA encoding a viral antigen. The mRNA is enclosed in lipid nanoparticles (LNPs) to protect it and help entry into human cells
2) Cellular Uptake & Translation - Host cells absorb the LNPs via endocytosis = mRNA is released into the cytoplasm, where ribosomes translate it into the viral protein.
3)Antigen Presentation - New viral protein is displayed on the cell surface via MHC class I (for cytotoxic T-cell activation) and MHC class II (for helper T-cell activation).
4) Immune Activation
B cells produce antibodies against the viral protein.
T cells (both cytotoxic and helper) are activated, boosting adaptive immunity.
5) memory B and T cells are formed
how are mRna vaccines manufactured ?
1) Vaccine target sequence is identified and cloned into a plasmid
2) Transformation, fermentation and plasmid isolation
3) Restriction enzyme linearises plasmid template
4) In vitro transcription generates mRNA from vaccine target DNA sequence
5) mRNA mixed with lipid nanoparticles
6) mRNA vaccine is produced
WARNING : nanoncages = have a protein and RNA base = question can be related to both
What are protein nanocages?
nanometer-sized particles made of protein subunits that self-assemble into symmetric structures
-Used in therapeutics
-Decreased immune response
-Used in drug delivery
What are mosaic vacccines?
They are active against multiple strains of a virus (AI is used to predict mutations to immunise for the future)
e.g. SpyTag/SpyCatcher system
What is CRISPR-Cas9 function?
a gene-editing tool that allows scientists to precisely cut and modify DNA at specific locations within an organism's genome by using a guide RNA to direct the Cas9 enzyme to the target sequence, essentially enabling the alteration or "editing" of genes at a desired location
Note:
-used to treat genetic disorders
-Very specific
How can we use CRISPR-Cas9 to treat viral infections e.g. hiv?
- Modification of receptors for viral entry
- Targeting pro-viral host factors
-Gain of function of restriction factors (proteins that are produced by the host to inhibit viruses from replicating)
-Deletion of integrated viral genome
Challenges of using CRISPR-Cas9 ?
-Long term for patients changing the patients proteins is risky as we don't know its full function
-Off target effects - CRISPR may cut unintended DNA sequences, leading to mutations that could cause diseases like cancer
-Efficiently delivering CRISPR components (Cas9 + guide RNA) into human cells is difficult.
-Viral vectors (e.g., AAVs) and nanoparticles are used but may cause immune responses or have size limitations.
-Not all target cells successfully integrate the desired edits, leading to a mixed population of edited and unedited cells (mosaicism)
-Ethical Concerns (Germline Editing)
Editing germline cells (sperm, eggs, embryos) can pass changes to future generations, raising ethical and safety concerns
-Invivo effectiveness due to immune system