PAG 1 - Moles determination
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
What equipment may we need for determining the amount in moles of a substance (PAG 1)?
Depends on which experiment we do.
- Heatproof mat
- Magnesium ribbon
- Crucible
- Pipeclay triangle
- Tripod
- Bunsen burner
- Balance
- Evaporating dish
- Measuring cylinder/gas syringe
- Conical flask
- Bung
Describe the method for determining the amount in moles of a substance (PAG 1).
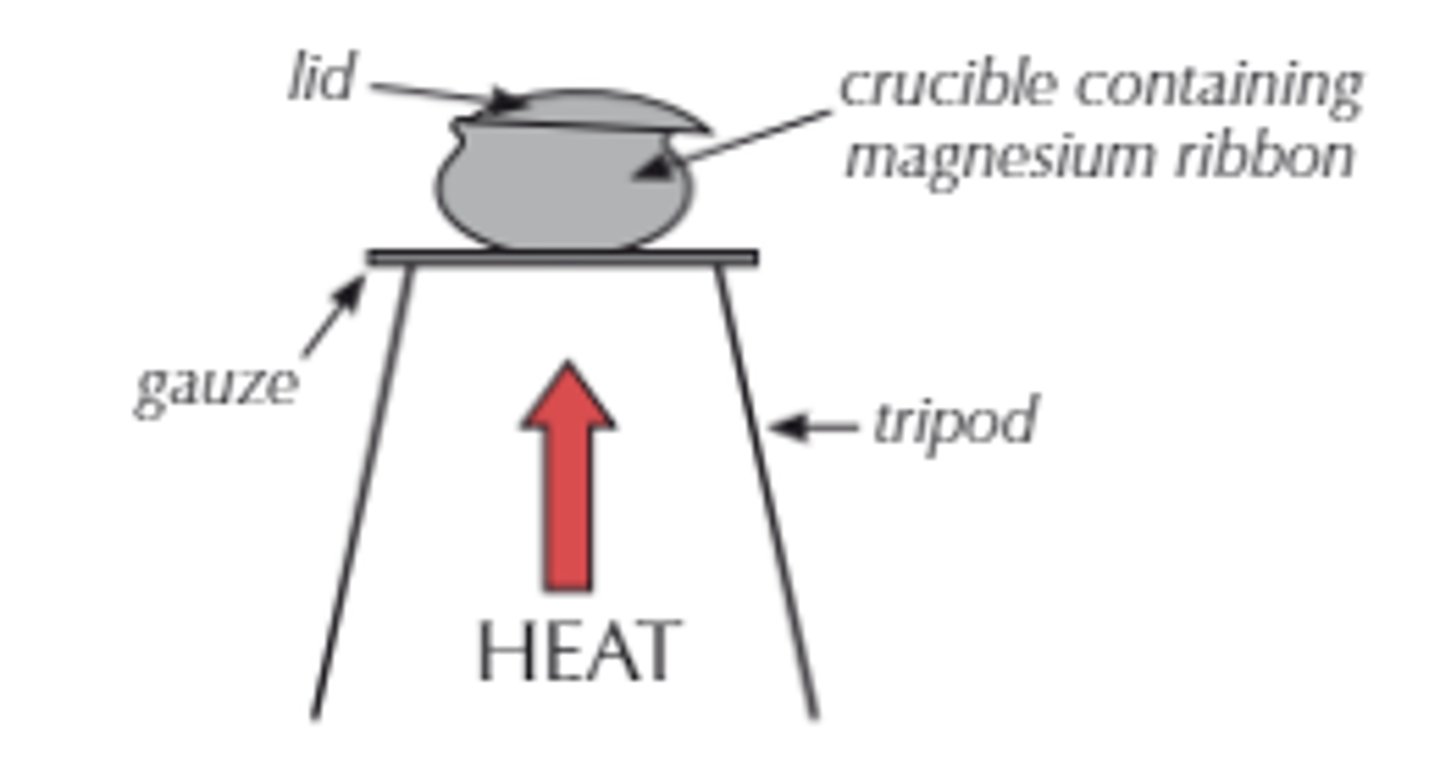
1. Set up equipment (see diagram below)
2. Measure the mass of the crucible and lid using a balance
3. Add the Mg ribbon and reweigh
4. Place the crucible on the pipeclay triangle and heat strongly
5. Lift lid a bit to allow air into the crucible
6. Let crucible cool and reweigh
7. This has made MgO
Diagram of experimental set up.
How to calculate the formula of magnesium oxide.
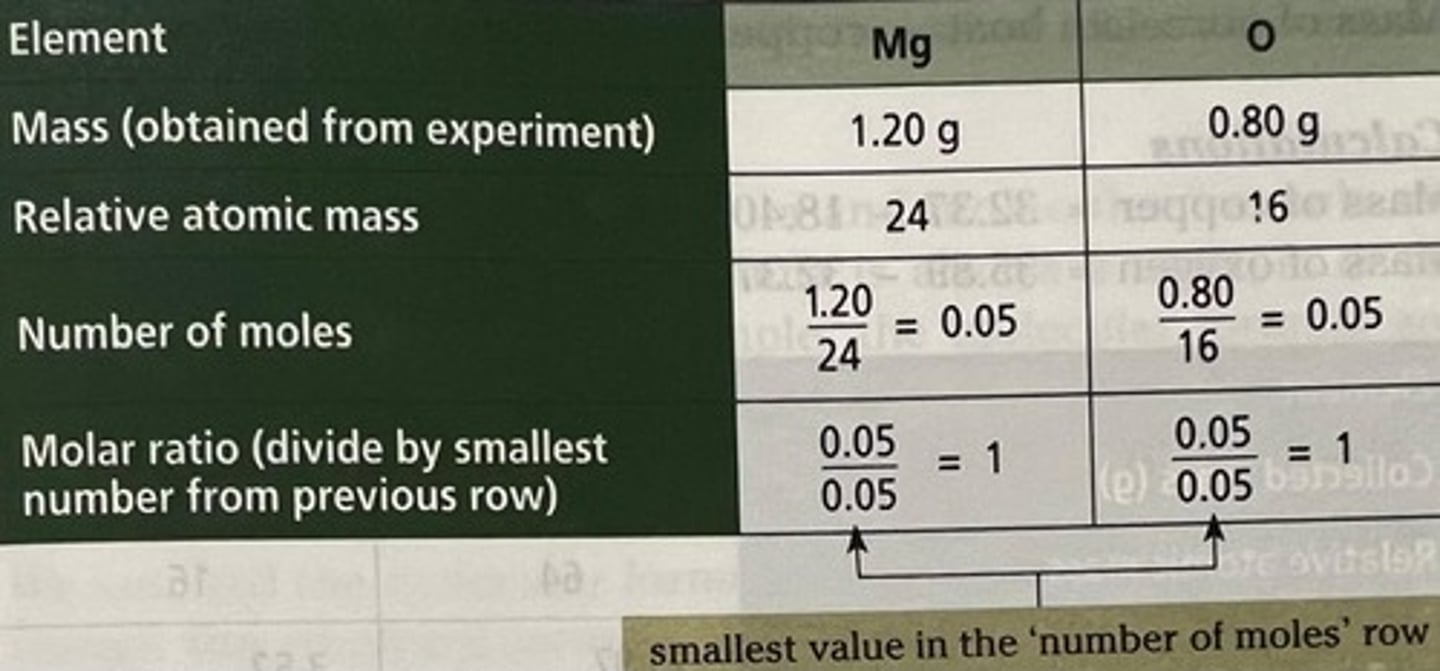
How do you calculate the moles of MgO?
Once you have the Mr and the mass, you can use the equation n=m/Mr
What are some potential sources of error in determining the amount in moles of a substance (PAG 1)?
- Not all the Mg reacted
- When the lid is lifted, some of the MgO may escape
How can the errors in determining the amount in moles of a substance (PAG 1) affect the results?
They would both yield a larger Mg:O ratio
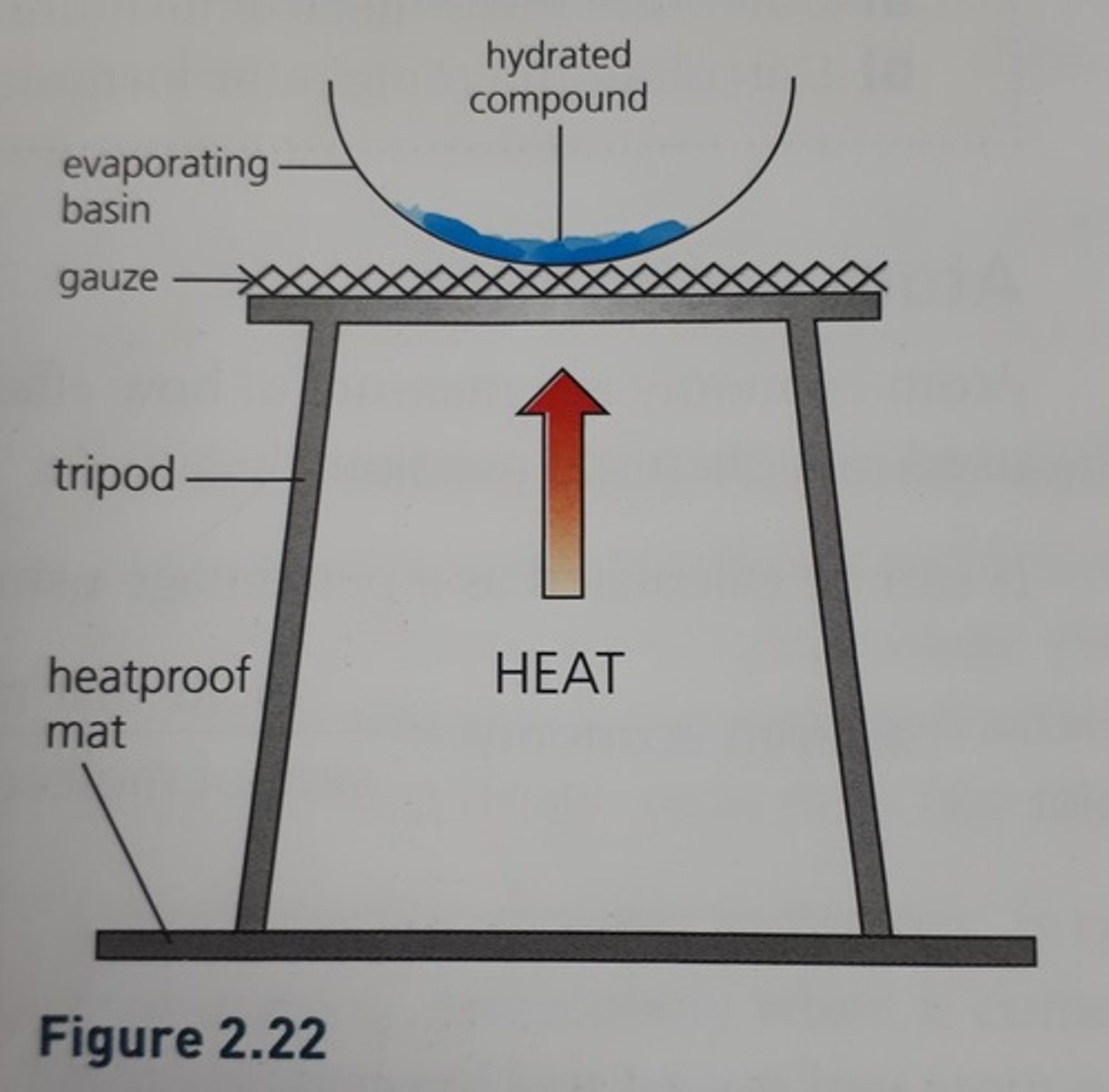
Describe the method for determining the amount in moles of a substance (PAG 1) if the compound is hydrated.
1. Weigh crucible/evaporating dish
2. Add hydrated compound and reweigh
3. Set up equipment as shown in diagram below
4. Heat until a constant mass (heat, cool, reweigh and repeat until the mass is constant)
5. There may be a colour change e.g. blue to white, steam given off or condensation at side of basin
6. The decrease in mass is the water lost
Heating to remove water of crystallisation diagram.
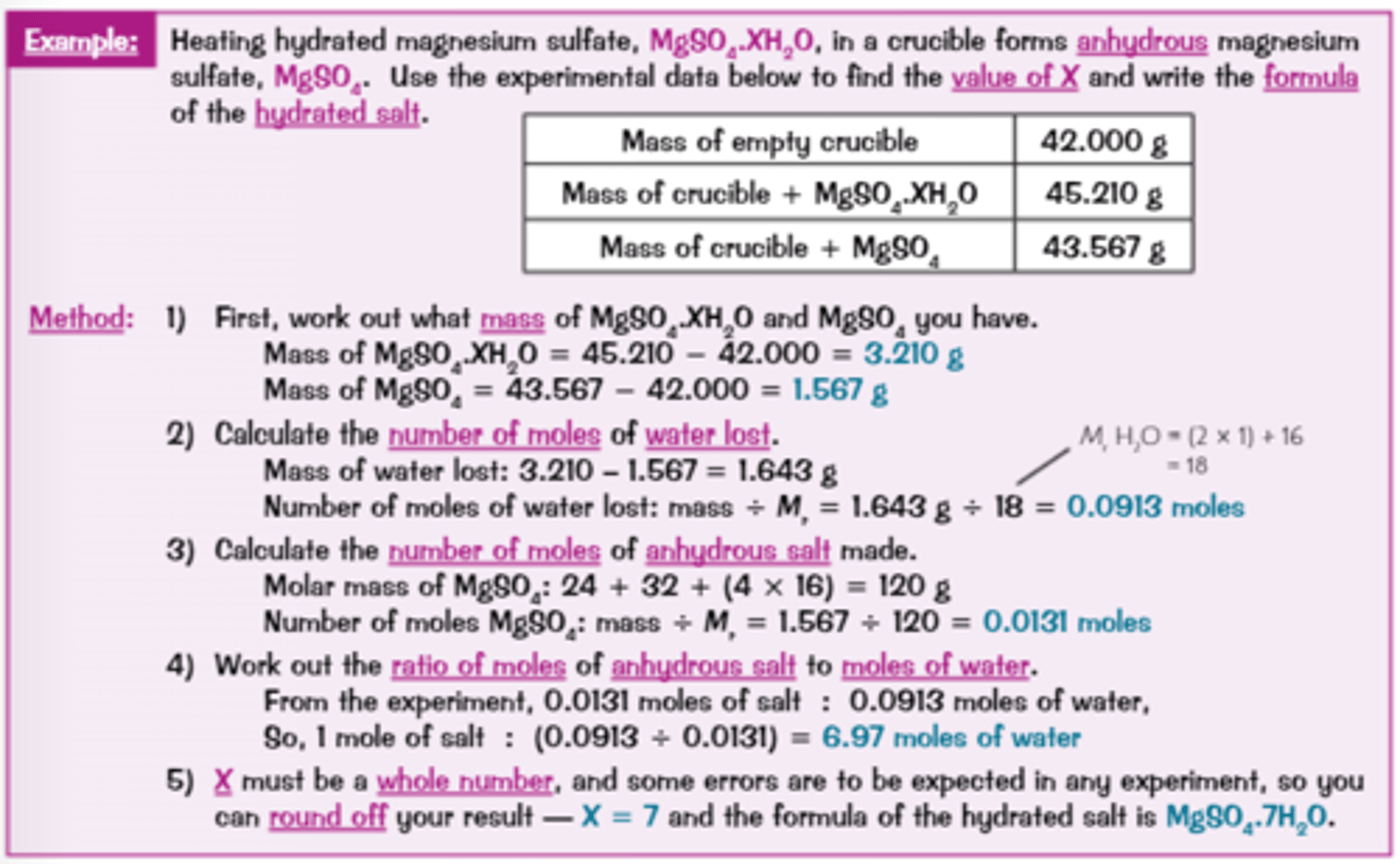
How do you calculate moles/formula of a hydrated compound using experimental results?
How do you prove a substance contains water of crystallisation?
Heat and hold blue cobalt chloride paper above it. It will turn pink if water is present
When can this experimental method of determining moles of a hydrated compound not work?
When the compound is decomposed by gentle heat e.g. nitrates
Describe the method for determining the amount in moles of a substance (PAG 1) if we are measuring production of a gas.
1. Set up the equipment as shown in the diagram below
2. Drop the Mg ribbon in the flask and stopper with the bung
3. Plug the bung tube into a gas cylinder
4. Record the initial volume of gas in the cylinder and the final volume of gas in the cylinder
Diagram to show how to measure the production of a gas.
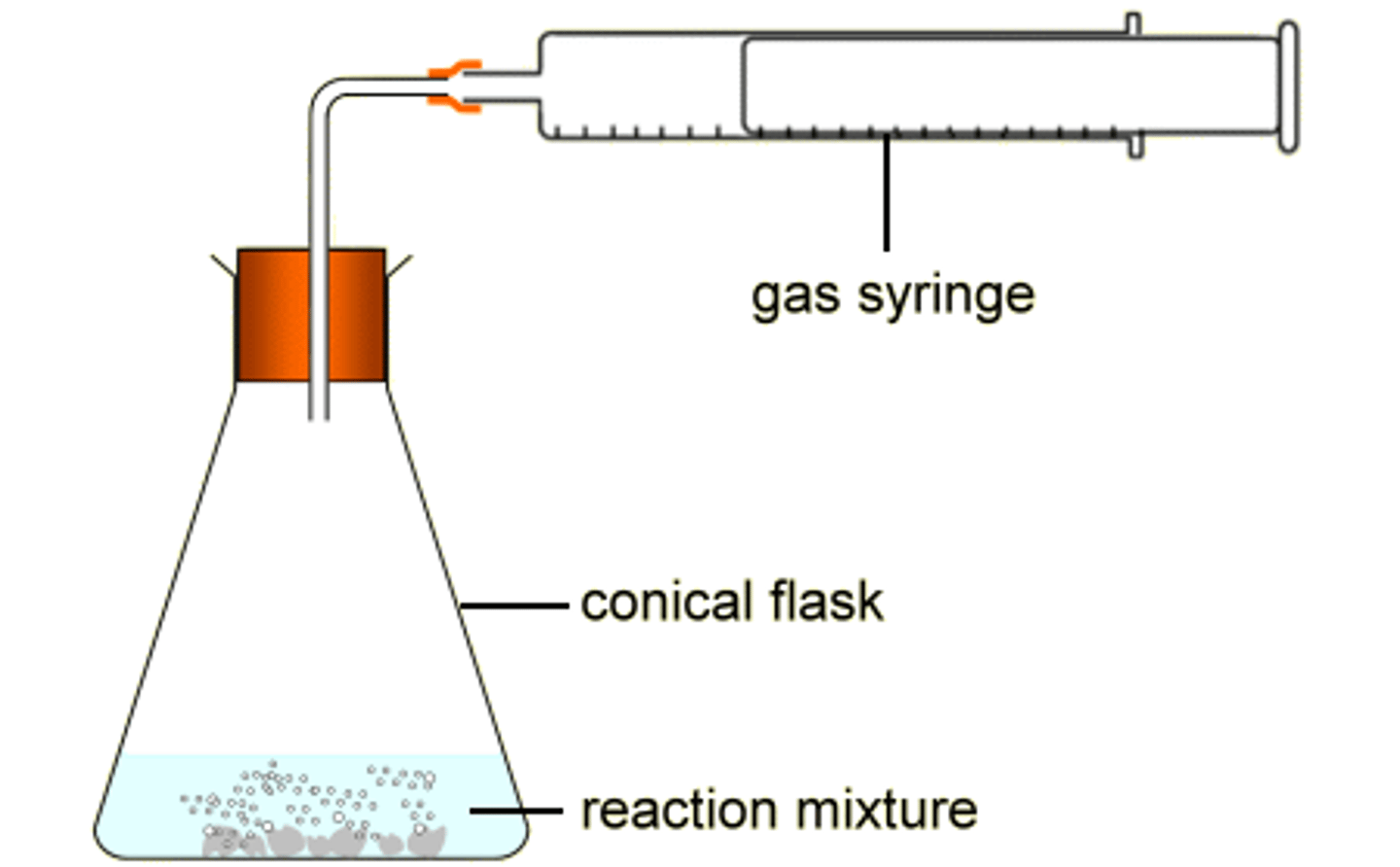
How do we calculate formula/moles from the volume of gas produced?
Use the overall reaction equation if given and the correct moles and gas equation.
What might be some sources of error when determining moles/formula using the volume of gas produced?
Some gas may escape before the bung is replaced