6 Signal Transduction
1/197
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
198 Terms
Define signal transduction
Signal transduction is the process of transmitting information from the extracellular environment into the cell.
Extracellular signals—also known as primary messengers or mediators—bind to specific cell surface receptors.
The binding event triggers a signaling cascade that ultimately leads to a biological response.
what are the methods of cellular signal transmission?
Endocrine signalling
Paracrine signalling
Autocrine signalling
Gap junctions
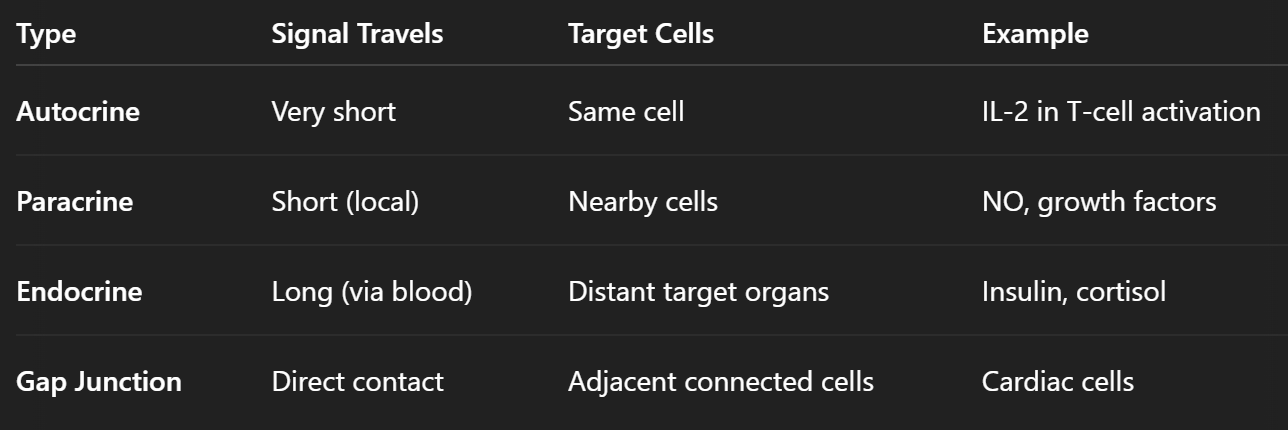
describe the mechanism, function and significance of autocrine signalling
Mechanism: A cell releases signalling molecules that bind to receptors on its own surface.
Function: Facilitates self-regulation and can reinforce a particular cellular response within the same cell.
Significance: Common in processes where feedback loops are needed for maintaining cell function or survival.
describe the mechanism, function and significance of endocrine signalling
Definition: Involves the release of hormones by specialized endocrine cells into the bloodstream, allowing the signal to travel long distances to reach target cells throughout the body.
Examples:
Insulin secreted by the pancreas affects glucose uptake in muscle and fat cells.
Thyroid hormones influence metabolism in virtually all tissues.
Characteristics:
Slow but long-lasting effects.
Very specific due to receptor binding on target cells.
Regulated through feedback loops (e.g., hypothalamic-pituitary axis).
describe the mechanism, function and significance of paracrine signalling
Definition: A signalling molecule is released by a cell and acts on neighbouring cells in the local environment.
Examples:
Growth factors like fibroblast growth factor (FGF) during wound healing.
Nitric oxide (NO) acting on nearby smooth muscle cells to cause vasodilation.
Characteristics:
Local and fast-acting.
Important in processes like inflammation, tissue repair, and development.
Signal is often rapidly degraded or taken up to limit the range.
describe the mechanism, function and significance of gap junctions
Definition: Specialized intercellular connections that allow direct communication between neighbouring cells.
Function: Enable the passage of ions, metabolites, and small signalling molecules.
Role in Tissue Coordination: Essential for synchronizing activities in tissues such as cardiac and smooth muscle.
Describe the Steps of Signal Transduction
Synthesis: The extracellular signalling molecule is synthesized by the signalling cell.
Release: The signalling molecule is secreted into the extracellular space.
Transport: The signal is transported through the extracellular medium to the target cell.
Reception: A specific receptor on the target cell binds the signalling molecule.
Response: Binding initiates changes in metabolism, function, or development.
Termination: The signal is removed or degraded, ending the cellular response and resetting the system.
Describe the Structural Organization of a Signalling Pathway
Extracellular Ligands: The signals that initiate the pathway.
Receptors: Proteins that recognize and bind the extracellular signal.
Intracellular Mediators: Include enzymes and second messengers that propagate the signal.
Second Messengers: Small molecules that amplify and distribute the signal within the cell.
Enzymes: Such as kinases and phosphatases that modify other proteins.
Adaptor Proteins: Facilitate the assembly of signalling complexes.
Effectors: The final targets that execute the cellular response.
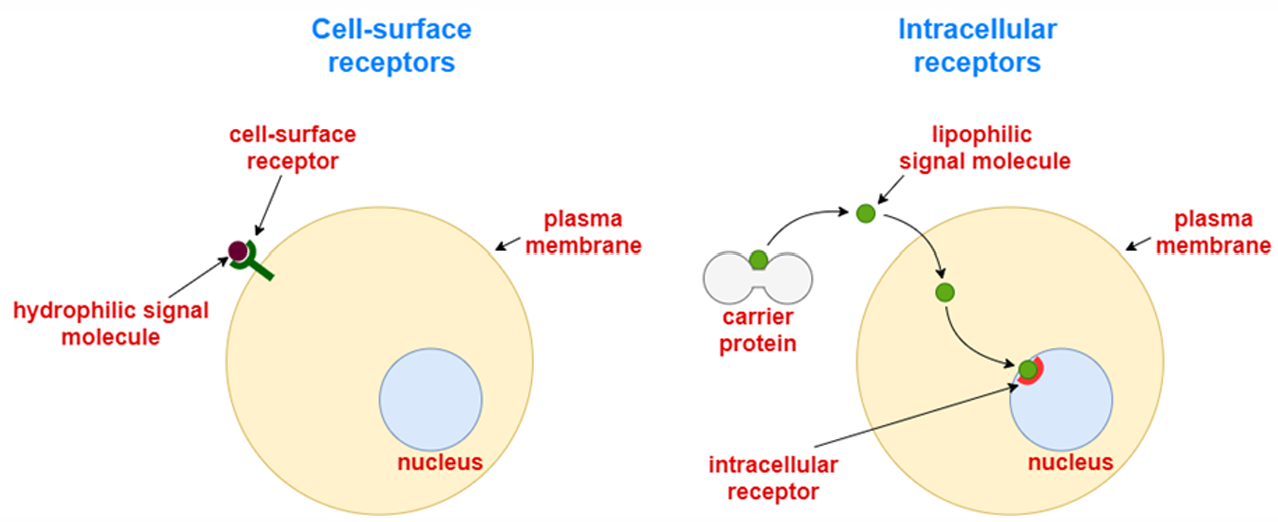
what are the 2 main types of ligands/extracellular signals?
1. Water-soluble ligands.
They cannot diffuse through the cell membrane (because it is hydrophobic).
Therefore, they bind to a ligand-binding site exposed on the extracellular region of the membrane receptor.
Examples of such ligands include amines, amino acids, peptides, and proteins.
2. Lipid-soluble ligands.
They can freely cross the cell membrane and bind to cytosolic receptors.
The ligand-receptor complexes then diffuse through the nuclear membrane and accumulate in the nucleus, where they modulate DNA transcription.
In this sense, these receptors are called nuclear receptors.
Examples of such ligands include steroid hormones (progesterone, estrogens, testosterone) and non-steroid hormones (thyroxine and triiodothyronine), vitamin D3, and retinoic acid.
what are the 2 main types of receptors?
Membrane (cell-surface) Receptors for water-soluble extracellular signalling molecules that cannot pass through the cell membrane
Intracellular Receptors for lipid-soluble extracellular signals that can freely cross the cell membrane. They are divided into type 1 (mainly in the cytoplasm) and type 2 (mainly in the nucleus)
define Latent Gene Regulatory Proteins
“Latent” gene regulatory proteins are transcription factors or co-regulators that are sequestered in an inactive state—often via proteolytic turnover or cytoplasmic retention—until a signalling event stabilizes and/or activates them, allowing nuclear translocation and target‐gene activation
define Scaffold Proteins
Scaffold proteins simultaneously bind multiple members of a signalling cascade, tethering them into complexes that enhance pathway specificity, speed, and localization
define Relay Proteins
Relay proteins simply pass the activated state from one component to the next (e.g., kinase to kinase), forming the core “linear” chain of a signalling pathway
define Bifurcation Proteins
Bifurcation proteins branch a single upstream signal into two or more distinct downstream pathways, enabling a single cue to generate multiple cellular outcomes
define Adaptor Proteins
Adaptor proteins possess interaction domains (e.g., SH2, SH3) and function as molecular bridges, linking specific signalling proteins without enzymatic activity of their own
define Amplifier Proteins
Amplifier proteins escalate signal strength by activating multiple downstream targets or by generating large numbers of small-molecule mediators (e.g., kinases, ion channels, cyclases)
define Transducer Proteins
Transducer proteins convert one form of signal into another—such as converting ligand-induced conformational change into kinase activation—thereby propagating the message in a new chemical form
define Integrator Proteins
Integrator proteins collect and integrate inputs from multiple, independent signalling pathways, synthesizing them into a unified downstream response
define Anchoring Proteins
Anchoring proteins (e.g., A-kinase anchoring proteins, AKAPs) tether key enzymes and effectors to specific subcellular locales, creating spatially constrained signalling “microdomains”
define Modulator Proteins
Modulator proteins regulate the activity or sensitivity of core signalling components—often via feedback loops or post-translational modifications—to fine-tune pathway output
define and describe the types of Molecular Intracellular Switches
Many intracellular signalling proteins act as switches, toggling between active and inactive states.
Protein Kinases/Phosphatases: Regulate activity through phosphorylation.
Protein Kinases: Enzymes that add phosphate groups to proteins (phosphorylation), often leading to activation.
Protein Phosphatases: Enzymes that remove phosphate groups (dephosphorylation), turning off signals.
Phosphorylation cascades can rapidly amplify the signal, leading to a significant cellular response. Sequential activation of protein kinases, where one kinase activates the next. A small initial signal is magnified, allowing for a robust and rapid cellular response. Critical for both signal amplification and eventual signal termination.
GTP-Binding Proteins: Function by binding and hydrolysing GTP. They possess GTPase activity, which allows them to hydrolyze GTP to GDP, thereby turning themselves off. These proteins toggle between:
Active State: Bound to GTP.
Inactive State: Bound to GDP.
Large Heterotrimeric G-Proteins: Typically associated with GPCRs, mediating signals from the cell surface.
Small Monomeric GTPases: Involved not only in signaling but also in processes like vesicular trafficking.
The dynamic switching is essential for both activating and deactivating the signalling cascade.
describe the role, function and interaction domains of adaptor proteins
Role: Serve as molecular docking platforms rather than having catalytic activity.
Function:
Complex Assembly: Help bring together different signalling molecules.
Mediating Crosstalk: Enable interactions between different signalling pathways.
Key Domains:
SH2 Domains: Bind to phosphotyrosine residues.
SH3 Domains: Bind to proline-rich sequences.
describe the mechanisms and importance of signals to turn off the transduction pathway
Importance of Deactivation: For the cell to be ready for new signals, the active signalling molecules must be inactivated.
Mechanisms:
Dephosphorylation: Removal of phosphate groups by phosphatases.
GTP Hydrolysis: Conversion of GTP to GDP in GTP-binding proteins.
Resetting the pathway to ensure controlled, timely responses and prevent overactivation.
What are the Characteristic Features of Signal Pathways?
Integration: Combining information from various signals.
The coordination and interaction of multiple signaling pathways.
Allows cells to combine different types of information to finely tune the cellular response.
Ensures that the cell’s response is balanced and context-specific.
Convergence: Different receptors can trigger a common downstream response.
Signals from different independent receptors can merge to activate a common effector.
This redundancy increases the robustness of the signaling system, ensuring that essential responses are activated even if one pathway is compromised.
Divergence: A single signal may activate multiple pathways.
A single extracellular ligand can activate multiple downstream effectors.
Leads to a variety of cellular responses from one signaling event.
Demonstrates how complex and versatile cellular responses can be, even from a single signal.
Crosstalk: Overlapping components allow for coordinated regulation across pathways.
Occurs when the same signaling molecules participate in more than one signaling pathway.
Allows for communication and coordination between different pathways.
Enhances the cell’s ability to respond to a dynamic and changing environment.
describe the role of protein acylation
Fatty Acid Acylation Activates Signalling Molecules Upon Translocation to the Cell Membrane
Post-Translational Modification: Fatty acid acylation attaches fatty acid chains to proteins.
Functions:
Membrane Anchoring: Helps anchor signaling proteins to the cell membrane.
Stabilization: Enhances protein-protein interactions.
Regulation: Modulates enzyme activities, particularly within mitochondria.
Mutations that prevent acylation can impair or reduce the biological function of these proteins.
Specific Example: Ras protein requires lipid modifications.
Farnesylation: A type of prenylation that adds a farnesyl group.
Acylation: Further modification that ensures proper membrane attachment.
These modifications are crucial for anchoring Ras to the Cell Membrane, where it can effectively participate in signal transduction.
what are the types of plasma membrane receptors?
lon channel-coupled
G-protein-coupled (guanine nucleotide-binding proteins)
Receptors with enzymatic activity:
Receptors with kinase activity: - with tyrosine kinase - with serine/threonine kinase - dual - specific
Receptors with protein phosphatase activity
Receptors with guanylate cyclase activity
Receptors associated with the functions of other tyrosine kinases
Death-receptors associated with apoptosis
Integrins (receptors regulating cell adhesion)
give the definition, function, significance and example of ion channel-coupled receptors
Receptors that form part of or are directly linked to ion channels.
Function: Change the permeability of the cell membrane by opening or closing ion channels in response to ligand binding.
Significance: Key in rapid signal transmission, particularly in neuronal communication
E.g. Acetylcholine Receptors
Structure: Comprised of several subunits (α₂, β, γ, δ) that assemble to form a functional ion channel.
Post-Translational Modification: The β, γ, and δ subunits can be phosphorylated by protein tyrosine kinases.
Classes:
Muscarinic receptors: Respond to muscarine.
Nicotinic receptors: Respond to nicotine.
Mechanism: Ligand binding triggers Na⁺ influx and K⁺ efflux, leading to depolarization and initiation of an action potential.
give the definition, function, structure and significance of G-Protein-Coupled Receptors (GPCRs)
A large and diverse group of receptors that activate intracellular G-proteins.
Function: Transduce extracellular signals into intracellular responses via second messengers.
Importance: Involved in many physiological processes, from sensory perception to hormonal responses.
Characterized by seven transmembrane alpha-helices.
Possess extracellular ligand-binding domains and intracellular domains that interact with G-proteins.
Structural Variations: Different classes have unique structural motifs that determine ligand specificity and signalling mechanisms.
GPCR Classification
Family A (Class I):
Largest group; contains conserved regions.
Notable feature: Palmitoylated cysteine at the C-terminus.
Examples: Rhodopsin, adrenergic, histaminergic, dopaminergic, muscarinic, and tachykinin receptors.
Family B (Class II):
Features a long N-terminal domain with six conserved cysteines forming disulfide bridges.
Examples: Glucagon, calcitonin, secretin, and PTH receptors.
Signalling: Often activates adenylate cyclase via Gs-protein.
Family C (Class III):
Binds neurotransmitters like glutamate or GABA.
Roles include the regulation of Ca²⁺ metabolism and taste.
Includes metabotropic glutamate receptors (mGluR) with allosteric sites that are potential drug targets for disorders such as Parkinson’s and schizophrenia.
General Overview of a G-Protein Signalling Pathway
Key Components:
Membrane Receptor: Activated by an extracellular ligand.
G-Protein: Activated by the binding of GTP.
Effector Protein: Receives signals from the G-protein (e.g., adenylate cyclase).
Second Messenger: Small molecules (e.g., cAMP) produced upon effector activation.
Upstream: Refers to the receptor.
Downstream: Refers to the effector and subsequent signaling events.
describe the composition and role of Heterotrimeric G-Proteins
Composition:
Consist of three subunits: α, β, and γ.
Roles of Subunits:
α-Subunit: Determines effector specificity; binds GTP.
β- and γ-Subunits: Involved in regulation of enzymes (e.g., phospholipase A₂, phospholipase C-β) and ion channels.
Signaling Diversity:
Different combinations of subunits allow for a variety of signaling outcomes.
Main G-Protein Families:
Gsα - activates adenylate cyclase and Ca2+ channels in all tissues. Increase cAMP. It is a substrate of cholera toxin.
Giα - activates PLC and PLA2 and K+ channels and inhibits cAMP, adenylate cyclase and Ca2+ channels
Gqα - activates PLC, found in all tissues. Increases DAG. IP3.
G12α - found in all tissues, regulates Nat/H+ antiport, electrical Ca2+ dependent channels, eicosanoid cell signals, and activates Rho proteins.
Other G-proteins: G11, G14, G15, G16 (G15 and G16 are in hematopoietic tissues).
describe the 3 main pathways of G-Protein signalling
The mammalian genome encodes ~20 different α-subunits, 6 β-subunits, and 12 γ-subunits.
Activation of Adenylate Cyclase (AC) ↑ cAMP
Hormones: ß1-Adrenergic agonists (adrenaline, noradrenaline), calcitonin, PTH, ADH, TSH, FSH, ACTH, LH, glucagon.
Activation of Phospholipase C (PLC) ↑ IP3 & DAG
Hormones: α1-Adrenergic agonists (adrenaline, noradrenaline), oxytocin, hypothalamic hormones, eicosanoids.
Inhibition of Adenylate Cyclase & Activation of Phosphodiesterase ↓ cAMP
Hormones: α2-Adrenergic agonists (adrenaline, noradrenaline).
Blockade of G-Protein Signalling
by Bacterial Toxins
Cholera Toxin:
Mechanism: ADP-ribosylates Gsα, preventing GTP hydrolysis.
Outcome: Persistent activation of Gs leads to increased cAMP and excessive secretion of Cl⁻ ions, resulting in severe diarrhea.
Pertussis Toxin:
Mechanism: ADP-ribosylates Giα, inhibiting its normal receptor interactions.
Outcome: Inhibits adenylate cyclase while activating other pathways (PLA₂, PLC), leading to disruption of normal hormone signaling.
What are the Receptors with Enzymatic Activity?
Not all receptors rely solely on secondary messengers.
Some have intrinsic enzymatic functions or are directly associated with enzymes.
Main Groups:
Receptors with protein kinase activity.
Receptors with protein phosphatase activity.
Receptors with guanylate cyclase activity.
According to their localization, two groups of protein kinases are distinguished:
Receptor kinases in the membrane.
Free protein kinases in the cytosol.
Depending on the amino acid they phosphorylate, protein kinases are divided into three groups:
protein tyrosine kinases
protein serine/threonine kinases
dual-specificity kinases
What are the Ligands for Receptor Tyrosine Kinases (RTKs)?
Nerve Growth Factor (NGF)
Platelet-Derived Growth Factor (PDGF)
Fibroblast Growth Factor (FGF)
Epidermal Growth Factor (EGF)
Insulin and Insulin-like Growth Factor-1 (IGF-1)
Ephrins (Eph)
Vascular Endothelial Growth Factor (VEGF)
Hepatocyte Growth Factor (HGF)
Stem Cell Factor (SCF, binds to c-Kit receptor)
Key Steps in RTK-Mediated Signalling Pathways
Activation Mechanism: Ligand binding induces dimerization and autophosphorylation (cross-phosphorylation) of tyrosine residues.
Signal Propagation: Phosphorylated tyrosine residues serve as docking sites for downstream signalling proteins.
Outcome: Initiates a cascade of phosphorylation events that ultimately affect gene expression and cellular behaviour.
describe the MAPK (Mitogen-Activated Protein Kinase) Pathway
Most ligands are cytokines, growth factors, hormones, and
neurotransmitters, selectively activating these cascades.
MAPK pathways operate through sequential phosphorylation, leading to transcription factor activation and gene expression regulation.
Mitogen-Activated Protein Kinases (MAPKs) are a family of Serine/Threonine kinases involved in key cellular processes: Cell proliferation, Cell differentiation, Cell migration, Cell death (apoptosis)
describe the types of Ras and its link to cancer
Ras Isoforms:
H-Ras, K-Ras, and N-Ras.
Common mutations in Ras proto-oncogenes (found in ~25% of human tumors) often lead to loss of GTPase activity.
Mutated Ras remains permanently active, promoting uncontrolled cell proliferation.
K-Ras mutations, for example, are prevalent in colorectal carcinomas.
Dysregulated Ras signaling is a key driver in oncogenesis.
give an example of Receptors with Protein Phosphatase Activity
Key Example – CD45:
First characterized transmembrane protein tyrosine phosphatase.
Critical in T-cell activation by regulating the activity of the Src-family kinase Lck.
Mechanism:
CD45 dephosphorylates the inhibitory tyrosine residue (Y505) on Lck, enabling its activation.
It can also dephosphorylate an activating residue (Y394) to maintain basal kinase activity.
Biological Role: Ensures proper regulation of T-cell receptor signaling and immune response.
give the function and example of Receptors with Guanylate Cyclase Activity
Function: Convert GTP to cyclic GMP (cGMP), a second messenger.
Examples: Receptors for natriuretic peptides (ANP), including ANPR-A, ANPR-B, and ANPR-C.
Physiological Relevance: Regulates cardiovascular functions such as vasodilation and blood pressure.
explain the difference between Primary vs. Secondary Messengers
Primary messenger: The extracellular signal (e.g., hormones, neurotransmitters).
Secondary mediators: Molecules that carry the signal inside the cell.
give the types and examples of secondary mediators
Cyclic Nucleotides:
cAMP: Activates protein kinase A (PKA).
cGMP: Activates protein kinase G (PKG).
Lipid Mediators:
Diacylglycerol (DAG): Activates protein kinase C (PKC).
Inositol-3-phosphate (IP3): Increases intracellular Ca²⁺ levels.
Additional lipids: Ceramide and sphingosine-1-phosphate.
Calcium and Calmodulin:
Elevations in Ca²⁺, along with calmodulin, activate Ca²⁺/calmodulin-dependent protein kinases (types I, II, and III).
Hormone-Receptor Complexes:
Example: Steroid hormones that directly affect gene expression.
describe the role, mechanism of action and receptor regulation of cAMP as a Key Second Messenger
Fundamental Role:
First identified second messenger, critical for transmitting extracellular signals.
Involved in compartmentalizing signals within the cell.
Mechanism of Action:
cAMP is synthesized from ATP and plays a pivotal role in regulating various cellular responses.
Selectively activates isoforms of protein kinase A (PKA) to modulate metabolism, gene transcription, and other processes.
Receptor Regulation:
Multiple G protein–coupled receptors (GPCRs) such as adrenergic, CRH, and glucagon receptors influence cAMP levels.
describe the production of Adenylyl Cyclase (AC) and cAMP
Enzymatic Conversion:
ATP is converted to cAMP by Class III adenylyl cyclases.
Isoforms of AC:
Transmembrane Adenylyl Cyclases (tmACs):
Humans have 9 types, regulated by GPCRs.
Play a critical role in responding to extracellular signals.
Soluble Adenylyl Cyclase (sAC):
Not regulated by G-proteins.
Directly activated by intracellular Ca²⁺ and bicarbonate (HCO₃⁻), functioning as a metabolic sensor.
describe GPCR Signalling & Adenylyl Cyclase Regulation
Signal Initiation:
Extracellular ligands (e.g., neurotransmitters, hormones, adrenaline) bind to GPCRs.
G-Protein Regulation:
Gs Protein (Stimulatory): Activates adenylyl cyclase to increase cAMP production.
Gi Protein (Inhibitory): Inhibits adenylyl cyclase, reducing cAMP levels.
Balances cellular responses by modulating the levels of the second messenger cAMP.
describe the mechanism of G-Protein Activation
Inactive State
G-proteins are heterotrimeric, consisting of α, β, and γ subunits, and are bound to GDP in the inactive state.
Membrane Anchoring Achieved through post-translational modifications: prenylation of βγ and myristoylation of the α-subunit.
Activation Process:
Ligand Binding: Causes a conformational change in the receptor.
GDP/GTP Exchange: GDP is replaced by GTP on the α-subunit.
Subunit Dissociation: The GTP-bound α-subunit dissociates from the βγ dimer and interacts with effector proteins (e.g., adenylyl cyclase).
describe Adrenaline Activation of the Adenylyl Cyclase System
Inactive State:
G-protein α-subunit is bound to GDP.
Activation:
Adrenaline binds to its receptor.
Triggers GDP-to-GTP exchange; the α-subunit activates adenylyl cyclase.
cAMP & PKA Activation:
Newly formed cAMP binds to the regulatory subunits of the PKA tetramer.
Catalytic subunits are released and phosphorylate various substrates, including proteins that translocate to the nucleus to affect gene expression
Initiates metabolic and transcriptional responses, such as glycogenolysis.
describe cAMP & Transcription Regulation
Role of CREB:
cAMP influences gene transcription primarily via the cAMP response element-binding protein (CREB).
Mechanism:
PKA phosphorylates CREB on a serine residue, enhancing its transcriptional activity.
Activated CREB binds to the cAMP response element (CRE) in DNA, leading to the transcription of genes involved in catabolic pathways (e.g., glycolysis, glycogenolysis, and lipolysis).
describe Adrenaline Activation of Glycogenolysis
Pathway: Adrenaline triggers a cAMP-dependent signaling cascade.
Mechanism:
Binding of adrenaline to its receptor elevates cAMP levels.
Activation of PKA leads to phosphorylation of key enzymes that catalyse the breakdown of glycogen.
Physiological Effect: Provides rapid energy mobilization during stress or increased energy demand.
list the enzymes regulated by phosphorylation by PKA and their pathway
Glycogen Synthase - glycogen synthesis
Phosphorylase Kinase - glycogen breakdown
Pyruvate Kinase - Glycolysis
Pyruvate Dehydrogenase - Pyruvate to acetyl-CoA
Hormone-sensitive Lipase - Triacylglyeride breakdown
Tyrosine Hydroxylase - Synthesis of DOPA, dopamine, norepinephrine
Histone H1 - Nucleosome formation with DNA
Histone H2B - Nucleosome formation with DNA
Protein phosphatase 1 Inhibitor 1 - Regulation of protein dephosphorylation
CREB - cAMP regulation of gene expression
PKA cosensus sequence - XR(R/K)X(S/T)B (B = hydrophobic amino acid)
describe the formation, isoforms and mechanism of Nitric Oxide (NO) signalling
NO is a short-lived gas & free radical, involved in various physiological & pathological processes
Synthesized from L-arginine via NO synthase (NOS), producing L-citrulline as a by product
NOS Isoforms:
nNOS (Type I, neuronal) & eNOS (Type III, endothelial)
Constitutively expressed (latent enzymes)
Require high Ca2+ levels for activation
iNOS (Type II, inducible)
Ca2+-independent due to strong binding to Ca2+/calmodulin
NO Mechanism:
Diffuses into neighboring cells → activates soluble guanylate cyclase (sGC).
Increases cGMP levels, regulating enzymes & ion channels.
L-arginine → L-citrulline + NO
describe the Regulation of eNOS Activity
eNOS Function: Endothelial nitric oxide synthase (eNOS) produces NO, a critical regulator of vascular tone.
Stimuli: Shear stress, acetylcholine, VEGF, bradykinin, estrogen, S-1P, H2O2, and angiotensin-II stimulate eNOS activity.
Regulatory Mechanisms:
Localization: eNOS is targeted to caveolae in the endothelial cell membrane via myristoylation and palmitoylation.
Inhibition: Caveolin-1 binds to and inhibits eNOS.
Activation:
Calmodulin (CaM) displaces Caveolin-1 when Ca²⁺ levels rise.
Heat shock protein 90 (Hsp90) and Akt-mediated phosphorylation further enhance eNOS activity.
Cellular Signaling Cascades Regulating eNOS Activity
Shear Stress:
Activates G-proteins leading to PI3K, PDK, and the cAMP pathway, which phosphorylate and activate eNOS.
Growth Factors & Hormones:
VEGF, estrogen, S1P, and bradykinin trigger PI3K/Akt and PLC-γ pathways, increasing intracellular Ca²⁺ and DAG.
Metabolic Stress:
ATP breakdown activates cAMP-dependent PKA, which phosphorylates eNOS.
Protein Interactions:
Proteins like Dynamin-2 and Porin interact with eNOS to promote its Ca²⁺-dependent activation.
Substrate Availability:
Efficient arginine uptake is necessary as arginine directly interacts with eNOS in caveolae.
describe the Biological Role of eNOS
NO Production: eNOS synthesizes nitric oxide (NO) in endothelial cells.
Physiological Effects of NO:
Acts as a potent vasodilator, helping lower blood pressure.
Inhibits platelet aggregation, leukocyte adhesion, and vascular smooth muscle proliferation.
Plays a role in preventing angiogenesis.
Dysregulation of eNOS activity is linked to vascular diseases such as atherosclerosis and hypertension.
Acetylcholine-mediated eNOS activation leads to NO release, which diffuses into smooth muscle cells to induce relaxation and increase blood flow.
Nitroglycerin induces vasodilation
describe the role of NO in the Relaxation of Smooth Muscle Cells
NO diffuses from endothelial cells into adjacent smooth muscle cells.
It activates soluble guanylate cyclase, converting GTP into cGMP.
Elevated cGMP levels induce smooth muscle relaxation and vasodilation.
This mechanism is the basis for the action of nitroglycerin, used to treat angina by dilating blood vessels.
Regulation: Phosphodiesterases (PDEs) degrade cGMP to GMP, ensuring that the signal is transient.
Sildenafil (Viagra) inhibits PDE5, preventing cGMP breakdown and prolonging its vasodilatory effects.
Describe the Extracellular Fate of Nitric Oxide
NO reacts non-enzymatically with O₂ and H₂O in the extracellular milieu.
Products: Nitrate (NO₃⁻) and nitrite (NO₂⁻).
Physiological significance: These stable anions serve as reservoirs for NO bioactivity and can be recycled back to NO under hypoxic conditions
NO-Mediated Toxicity & Peroxynitrite Formation
NO + O₂⁻ → Peroxynitrite (ONOO⁻)
Peroxynitrite effects:
DNA damage: Causes strand breaks and base modifications leading to fragmentation.
Lipid peroxidation: Initiates free-radical chain reactions in membranes, compromising integrity and fluidity.
Overproduction of ONOO⁻ is implicated in inflammatory diseases, neurodegeneration, and ischemia–reperfusion injury.
Mitochondrial Impact of Peroxynitrite
Targets within the Electron Transport Chain (ETC):
Complexes I–IV are susceptible to nitration and oxidation, impairing electron flow and ATP synthesis.
Effect on MnSOD (Mitochondrial Superoxide Dismutase):
ONOO⁻ inactivates MnSOD, diminishing mitochondrial antioxidant defense.
Enhanced ROS production: Accumulation of O₂⁻ and H₂O₂ exacerbates oxidative stress, triggering mitochondrial permeability transition and apoptosis.
Regulation of Protein Activity by S-Nitrosylation
NO covalently modifies thiol (–SH) groups on cysteine residues to form S-nitrosothiols (RSNOs).
Alters protein conformation, activity, localization or interactions—akin to phosphorylation.
S-nitrosylation of ion channels, receptors, and enzymes modulates cardiovascular tone, neurotransmission, and immune responses.
Stability & Reversibility of S-Nitrosothiols
Chemical Lability: RSNO bonds are unstable — sensitive to light and oxidative environments, leading to spontaneous NO release.
Enzymatic Denitrosylation:
Thioredoxin/Thioredoxin Reductase (Trx/TR) System: Catalyzes removal of the –NO group, restoring the free thiol and terminating the nitrosylation signal.
Provides dynamic control over S-nitrosylation-dependent signaling pathways.
describe the the role of iNOS and NO in Innate Immunity
Inducible Nitric Oxide Synthase (iNOS): Expressed in macrophages in response to infection or inflammatory signals.
Role in Immunity:
High levels of NO are produced to combat pathogens and tumor cells.
NO, in conjunction with superoxide, acts as a potent antimicrobial and cytotoxic agent.
Microbial components such as bacterial and fungal cell wall elements stimulate iNOS expression.
Activation of iNOS Expression
1. Bacterial & Fungal Cell Wall Components
LPS (lipopolysaccharides) from Gram-negative bacteria bind to LBP (LPS-binding protein).
SOCS1 (Suppressor of Cytokine Signaling 1) inhibits the signaling cascade triggered by LPS.
This serves as a regulatory mechanism to prevent overactivation of the immune response and limit excessive NO production.
CD14 (LPS receptor) on macrophages/neutrophils interacts with TLR4 (Toll-like receptor 4) and MD2.
This triggers intracellular signaling cascades via adaptor proteins.
TRAF6 & p38 are activated, leading to IKK (IκB kinase) phosphorylation.
NF-KB is released, translocates to the nucleus, and induces iNOS transcription.
Normally, NF-κB is trapped in the cytoplasm because it is bound to a protein called IκB (inhibitor of NF-κB).
IKK phosphorylates (adds phosphate groups to) IκB.
Phosphorylated IκB is marked for destruction by the proteasome.
Once IκB is destroyed, NF-κB is free to move into the nucleus.
In the nucleus, NF-κB turns on genes related to inflammation, immune response, and cell defense.
2. Cytokines from Infected Cells
Interferons (IFNs) exhibit antiviral, immunostimulatory, antiproliferative, and antitumor effects.
IFN-α - secreted by leukocytes.
IFN-ß - secreted by fibroblasts.
IFN-γ - produced by lymphocytes, activates JAK-STAT signaling.
Leads to IRF1 (Interferon Response Factor-1) synthesis, stimulating iNOS mRNA transcription.
give the Calcium Concentration Ranges
Extracellular Ca²⁺: Approximately 5 mmol/L.
Free intracellular Ca²⁺: Ranges from 0.1 to 10 μmol/L.
Organelle-bound Ca²⁺ (e.g. mitochondria and ER): Ranges from 1 to 20 μmol/L
describe the mechanisms for Maintaining Low Cytosolic Ca²⁺
Ca²⁺-ATPase: Enzymes such as SERCA (in the ER) and PMCA (at the plasma membrane) pump Ca²⁺ out of the cytosol using ATP hydrolysis.
Ca²⁺-Binding Proteins:
Calbindin in the cytosol.
Calreticulin in the ER
both act as buffers
Na⁺/Ca²⁺ Exchanger (NCX):
Particularly active in excitable cells (e.g., muscle and neurons).
Uses the Na⁺ electrochemical gradient to export Ca²⁺ when intracellular levels rise significantly.
Ca²⁺-Mediated Signalling Pathway in Excitable Cells
in Neurons, Muscle, Pancreatic Endocrine Cells
Depolarization: Opens voltage-dependent Ca²⁺ channels (VDCC).
Triggers neurotransmitter and hormone secretion.
Induces muscle contraction.
Example: In pancreatic β-cells, the closure of K⁺-ATP channels causes membrane depolarization that opens VDCC.
Ca²⁺-Mediated Signalling Pathway in Non-Excitable Cells
Receptor-Ligand Binding: Activates Ca²⁺ entry through voltage-independent Ca²⁺ channels (VICC).
IP₃ Pathway:
Activation of receptors generates inositol trisphosphate (IP₃).
IP₃ binds to ER receptors, releasing Ca²⁺ into the cytosol.
Short-Term Cellular Responses of Ca²⁺ as an Intracellular Second Messenger
Excitation-Contraction Coupling: Critical for skeletal and cardiac muscle function.
Stimulus-Secretion Coupling: Essential for endocrine and neuronal cells.
Mechanisms Involved:
Post-translational modifications (for example, phosphorylation of ion channel subunits).
Activation of Ca²⁺-dependent kinases such as CaM-kinase II and protein kinase C (PKC).
Stimulation of Ca²⁺-sensitive adenylyl cyclases that lead to cAMP signalling.
Long-Term Cellular Responses of Ca²⁺ as an Intracellular Second Messenger
Gene Expression: Influences cell proliferation, differentiation, apoptosis, and neuronal adaptation.
Regulation:
Involves Ca²⁺-regulated proteins such as hormones, neuropeptides, ion channels, and oncogenic proteins.
Ca²⁺-responsive gene elements modulate transcription.
Low cytosolic/ER Ca²⁺ can inhibit cell proliferation.
Calmodulin (CaM) and Its Cellular Functions
CaM is a Ca²⁺-dependent protein predominantly located in the cytosol.
Structurally and functionally analogous to troponin C found in muscle tissue.
Frequently acts as a regulatory subunit within various protein complexes.
Modulates the activity of several kinases, enzymes involved in cyclic nucleotide metabolism, and proteins responsible for ion transport.
Regulated Cellular Processes:
Regulation of the actin-myosin complex critical for smooth muscle contraction.
Control over microfilament-mediated activities, including:
Cell motility.
Mitosis.
Granule exocytosis.
Endocytosis.
Additional Ca²⁺-Binding Proteins Mentioned:
Annexins, sorcin, light myosin chains, beta-actin, calcineurin, and calpain are listed as other proteins that bind calcium.
List the Enzymes Regulated by Ca²⁺/Calmodulin
Cyclases:
Adenylate cyclase and guanylate cyclase, which synthesize cyclic nucleotides.
Protein Kinases:
Ca²⁺-dependent protein kinase, myosin kinase, cAMP-dependent protein kinase.
ATPases and Phospholipases:
Ca²⁺/Mg²⁺-ATPase, phospholipase A2.
Other Kinases & Phosphorylases:
Ca²⁺/phospholipid-dependent protein kinase, phosphorylase kinase.
Phosphatases and Other Enzymes:
Phosphoprotein phosphatase 2B.
Phosphodiesterase (breaks down cyclic nucleotides).
Metabolic enzymes such as pyruvate carboxylase, glycerol-3-phosphate dehydrogenase, pyruvate dehydrogenase, glycogen synthase, and pyruvate kinase.
Key Pathways Producing Lipid Mediators
Phospholipase A2 (PLA2) Route:
Involves the release of arachidonic acid (AA) from membrane phospholipids.
Phosphatidylinositol Route:
Involves the generation of inositol phosphates (through the phospholipase C pathway) and subsequent signals.
Sphingomyelin System:
Leads to the production of signalling molecules such as ceramide and sphingosine-1-phosphate (S1P).
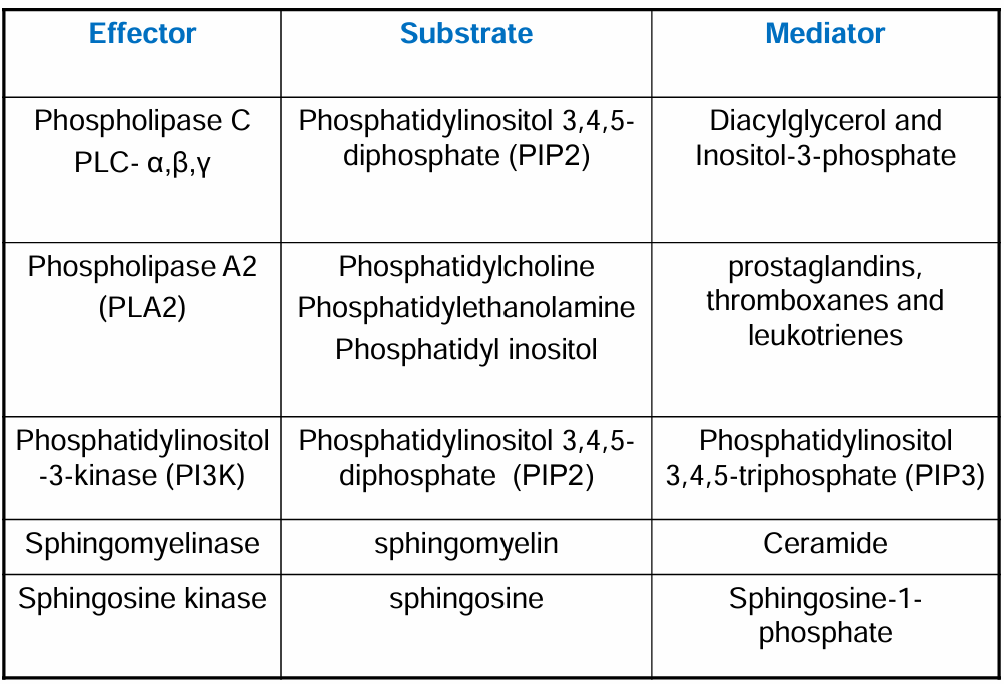
Arachidonic Acid Pathway & Lipid Mediators
Arachidonic Acid (AA): A polyunsaturated fatty acid released from membrane phospholipids through PLA2 activity.
Cyclooxygenase (COX) Pathway:
Isoforms:
COX-1: Constitutively expressed.
COX-2: Inducible under inflammatory conditions.
Converts AA into prostaglandins, prostacyclins (PGI₂), and thromboxanes (TXA).
Lipoxygenase (LOX) Pathway:
AA is converted to 5-HPETE.
5-HPETE is further metabolized to Leukotriene A₄ (LTA₄).
Leukotriene Derivatives:
LTB₄: Promotes inflammation by inducing chemotaxis and degranulation of polymorphonuclear leukocytes.
LTC₄, LTD₄, LTE₄: Involved in vasoconstriction and bronchospasm, playing roles in asthma and anaphylaxis.
Lipoxins, which are also derived from AA, act as anti-inflammatory mediators
Phosphatidylinositol Pathway of Phospholipase C
Involves cleavage of membrane phospholipids to generate diacylglycerol (DAG) and inositol-3-phosphate.
Plays a key role in cellular signalling via second messengers.
Phosphatidylinositol-3 Kinase (PI3K) Pathway
PI3K is a Heterodimer - Comprised of 2 subunits
a regulatory subunit (p85, 85 kDa, contains SH2 domains, interacts with tyrosine-phosphorylated receptors or PTKs)
a catalytic subunit (p110, 110 kDa, enzymatic function, phosphorylates substrates.s
Phosphorylates phosphatidylinositols at the 3′ position on the inositol ring.
Activation: Triggered by receptor tyrosine kinases (RTKs) such as those for PDGF, EGF, Insulin, IGF-1, HGF, and NGF.
Downstream Signaling Events:
PI3K generates PIP3 (Phosphatidylinositol 3,4,5-triphosphate)
PIP3 activates PDK1, which binds to the PH domain of PKB/Akt
Cellular Outcomes: Regulation of adhesion, chemotaxis, apoptosis, secretion, glucose transporter translocation, platelet activation, and reorganization of the cytoskeleton.
Regulation by PTEN:
PTEN (Phosphatase and Tensin Homolog) dephosphorylates PIP3 back to PIP2.
This activity serves as a negative regulatory mechanism, reducing Akt activation and modulating cell survival and proliferation.
Ligand binding to membrane receptors activates PI3K
PI3K phosphorylates PIP2 (PI(4,5)P) → forms PIP3 (PI(3,4,5)P)
PIP3 recruits PDK1 to the plasma membrane
PDK1 phosphorylates and activates AK1 (PKB), regulating various cellular processes
PTEN (Lipid Phosphatase) dephosphorylates PIP3 → increases PIP2 levels
This reduces AKT activity, modulating cell signaling.
mTOR (Mammalian Target of Rapamycin)
mTOR: A serine/threonine kinase that functions as a central regulator in the PI3K/Akt pathway.
Roles: Controls cell growth, proliferation, metabolism, survival, and protein synthesis.
Dysregulation: Overactivity of mTOR can lead to tumorigenesis by promoting anabolic processes while inhibiting autophagy.
mTOR Inhibitors: Rapamycin (Sirolimus) and its analogs are employed both as immunosuppressants (to prevent transplant rejection) and as targeted cancer therapies.
mTOR Complexes:
mTORC1:
Rapamycin-sensitive
Sensor for nutrients and energy status.
Activated by PI3K/Akt in response to insulin, IGF-1, IGF-2, PDGF, EGF, HGF.
Requires amino acids, ATP, oxygen; inhibited by low ATP (AMPK activation) & hypoxia.
Regulates protein and lipid metabolism and suppresses autophagy.
Target in cancer therapy (Rapamycin analogs).
mTORC2:
Regulation of the cytoskeleton and cell survival (e.g. via Akt Ser473 phosphorylation)
Does not respond to nutrients
Ceramide in Apoptosis Regulation
Role as a Secondary Messenger: Ceramide is crucial in the initiation phase of apoptosis.
Extrinsic Apoptotic Pathway:
Mediated by tumor necrosis factor (TNF) receptors and the formation of a death domain (DD) signalling complex that activates transcription via the JNK pathway
Intrinsic Apoptotic Pathway:
Alters mitochondrial function in response to stress signals (UV radiation, oxidative stress, ionizing radiation, heat shock)
Some tissues may undergo apoptosis with minimal ceramide contribution.
In disorders such as Niemann–Pick disease (associated with a sphingomyelinase defect), a deficiency in ceramide leads to diminished apoptosis.
Sphingosine-1-Phosphate (S1P) Signalling
Synthesis and Activation:
Formation of S1P: Sphingosine is phosphorylated by sphingosine kinase 1 (SphK1).
Stimulatory Factors for SphK1: Growth factors, chemokines, oxidative LDL, ATP, acetylcholine, vitamin D3, and several cytokines (such as PDGF, VEGF, TNF-α, EGF, and basic fibroblast growth factor).
Autocrine/Paracrine Action: S1P is secreted and acts via its G-protein-coupled receptors (S1PRs) on the cell surface.
Receptor Cross-Talk: Activation of the PDGF receptor can lead to subsequent activation of FAK, Src, and Rac; these signals promote cell migration.
Downstream Pathways:
Extracellular S1P: Activates S1PR1, which in turn triggers the JAK2/STAT3 pathway; this pathway regulates the expression of IL-6 and S1PR1.
Intracellular S1P: Can activate NFκB, which feeds back to regulate SphK1 expression.
The sustained activation through the S1P-STAT3-NFκB loop may drive cell transformation and tumor progression.
give the definition and function of a hormone
Any substance in the body that transmits a signal and leads to a change at the cellular level (a change in cellular behaviour)
Hormones are biochemical messengers produced by endocrine glands or cells and released into the bloodstream.
They trigger specific changes in target cells that possess the appropriate receptors.
Classification by solubility:
Hydrophobic - Cross membranes easily
Hydrophilic - Require membrane receptors
Classification of hormones and signalling molecules based on their chemical structure
Peptide and Protein Hormones:
Examples: Insulin, Glucagon, Growth Hormone (GH), Adrenocorticotropic Hormone (ACTH).
Steroid Hormones:
Examples: Cortisol, Aldosterone, Estrogens (e.g., Estradiol), Androgens (e.g., Testosterone).
Amino Acid-Derived Hormones:
Examples: Thyroid Hormones (T3, T4), Catecholamines (e.g., Epinephrine, Norepinephrine, Dopamine), Melatonin.
Lipid-Derived Hormones (Eicosanoids):
Examples: Prostaglandins, Leukotrienes, Thromboxanes.
Glycoprotein Hormones:
Examples: Luteinizing Hormone (LH), Follicle-Stimulating Hormone (FSH), Thyroid-Stimulating Hormone (TSH).
Gaseous Signaling Molecules:
Examples: Nitric Oxide (NO), Carbon Monoxide (CO), Hydrogen Sulfide (H₂S).
Describe the main groups of hormones by Action and Properties
Group I Hormones:
Types: Steroids, iodothyronines (e.g., thyroid hormones), calcitriol, retinoids.
Solubility: Lipophilic.
Transport: Often require binding to plasma transport proteins.
Plasma Half-Life: Generally long (hours up to days).
Receptor Localization: Act on intracellular receptors.
Secondary Mediators: Typically do not require classic secondary messengers.
Group II Hormones:
Types: Polypeptides, proteins, glycoproteins, catecholamines.
Solubility: Hydrophilic.
Transport: Circulate freely in plasma without specific binding proteins.
Plasma Half-Life: Short (minutes).
Receptor Localization: Act via receptors on the plasma membrane.
Secondary Mediators: Often signal through systems such as cAMP, cGMP, Ca²⁺, lipid mediators, or kinase cascades.
Pituitary Gland & Hypothalamic Control
Pituitary Anatomy:
Composed of two lobes:
Adenohypophysis (Anterior Lobe).
Neurohypophysis (Posterior Lobe).
Hypothalamic Influence:
The hypothalamus synthesizes releasing hormones that are secreted into the portal circulation of the anterior pituitary
These releasing hormones activate intracellular signalling pathways (involving Ca²⁺, IP₃, and PKC) leading to exocytosis of pituitary hormones
The released tropic hormones regulate growth and activity in target endocrine glands, forming the basis of the hypothalamic–pituitary axis.
Molecular Characteristics of Trophic - Anterior Pituitary Hormones
The anterior pituitary secretes tropic hormones which regulate growth and secretion in other endocrine glands.
Glycoprotein Hormones (TSH, FSH, LH):
Composed of two peptide chains:
β-chain: Specific and Biologically active
α-chain: Identical in all three hormones.
Growth Hormone & Prolactin:
Structurally similar; both are gene duplicates (~200 amino acids) with similar sequences.
POMC-Derived Hormones:
Precursor molecule is processed via specific proteolysis to yield multiple biologically active hormones such as ACTH, β-endorphin, β-lipotropin, α-MSH, and β-MSH.
Hypothalamic-Pituitary-Thyroid Axis
Hypothalamus: Produces thyrotropin-releasing hormone (TRH).
TRH is transported via portal circulation to the pituitary
Pituitary: In response to TRH, the adenohypophysis secretes thyroid-stimulating hormone (TSH)
Thyroid Gland: TSH stimulates the thyroid to produce and release thyroid hormones (T4 and T3).
The hypothalamic TRH prompts intracellular signalling in the pituitary, preparing the gland to release TSH, which then influences thyroid function.
TRH
Structure: Modified tripeptide, synthesized as a prohormone.
Stimulates TSH subunit biosynthesis & glycosylation.
Receptor downregulation via negative feedback from TSH & thyroid hormones.
Intracellular Signalling Pathways of TRH
TRH receptor (TRH-R) activation → Stimulates phospholipase C (PLC).
InsP3 production → Triggers Ca2+ release from intracellular stores → TSH exocytosis
Key signalling cascades:
Ca2+/Calmodulin-dependent kinase (Ca2+/CamKin)
Protein kinase C (PKC)
MAPK pathway
Transcription factors activated: CREB, AP-1, Elk-1.
TSH Receptor and Signal Transduction
Structure of the TSH Receptor (TSHR): Glycoprotein receptor with three domains:
Extracellular domain: Binds thyroid-stimulating hormone (TSH).
Transmembrane domain: Spans the plasma membrane (7-transmembrane G protein-coupled receptor—GPCR).
Intracellular (cytoplasmic) domain: Interacts with G proteins to initiate intracellular signaling cascades.
TSH binds to the extracellular domain of TSHR → triggers a conformational change → activates G proteins (mainly Gs and Gq subtypes).
Intracellular Signaling Pathways Activated:
cAMP pathway (via Gs protein): Activates adenylyl cyclase → ↑ cAMP → activates PKA → Stimulates transcription of genes involved in thyroid hormone synthesis and thyrocyte proliferation
Phospholipase C pathway (via Gq protein): Activates PLC → ↑ IP₃ (releases Ca²⁺ from ER) and DAG (activates PKC → modulates protein phosphorylation and secretion)
Key Cellular Effects of TSH:
Stimulates:
Iodide uptake via sodium-iodide symporter (NIS).
Thyroglobulin (Tg) synthesis and iodination.
Formation of iodothyronines (T3, T4) within the colloid.
Proteolysis of thyroglobulin → releases T3 and T4 into circulation.
Peripheral deiodination of T4 to active T3 (modulation of local thyroid hormone levels).
Promotes:
Thyroid follicular cell growth and division (trophic effect).
Negative Feedback Regulation of T3/T4:
Suppress TSH gene expression and secretion at the pituitary level.
Act through thyroid hormone receptors (TRs) in the pituitary to:
Inhibit TSH β-subunit transcription.
Reduce glycosylation and processing of TSH.
This feedback ensures homeostatic control of thyroid hormone levels
Thyroid Hormones (T4 & T3) Production & Regulation
Synthesis:
Thyroglobulin Iodination:
Thyroid hormones are produced by iodinating tyrosine residues within thyroglobulin (Tg), which is then stored in the colloid and hydrolyzed to release T4 and T3.
T4 (thyroxine) is produced in larger amounts compared to T3 (triiodothyronine)
Converstion by Deiodination:
T4 is converted to the more active T3 through deiodination processes.
Depending on the location (thyroid itself, target tissues, or peripheral tissues), different deiodination pathways modify T4 into active T3 (by 5’ deiodination) or the inactive reverse T3 (rT3, by 5-deiodination).
Secretion:
T4 and T3 are released via Tg hydrolysis (colloid droplets)
Iodide Recycling:
Iodide is recovered for reuse after hormone release.
Daily Tg turnover
100 micrograms released unchanged
only 25 micrograms hydrolysed to meet T4 demand
Thyroid Hormone Metabolism: Deiodinases
Type I Deiodinase (D1):
Catalyzes both 5’ (activating) and 5-deiodination (inactivating) reactions.
Found in liver, kidney, thyroid, pituitary, and CNS.
Exhibits a high Km for T4, producing both T3 and rT3; activity is increased in hyperthyroidism.
Type II Deiodinase (D2):
Catalyzes 5’-deiodination exclusively to produce active T3.
Present in the brain, brown adipose tissue, placenta, and pituitary.
Has a lower Km than D1, helping to maintain intracellular T3 levels; its activity can increase in hypothyroidism.
Type III Deiodinase (D3):
Catalyzes 5-deiodination, effectively inactivating T4 to rT3.
Mainly found in the brain and placenta to prevent excess T3 accumulation in sensitive tissues.
Metabolism/Degradation of T3 and T4
T4 metabolism:
Sulfation, decarboxylation, deamination, glucuronidation.
Liver: T4 undergoes deamination & decarboxylation → forms TETRAC (tetraiodothyroacetate).
T3 metabolism:
Sulfation (T3S).
Conversion to TRIAC (triiodothyroacetate).
Half-life in serum:
T4 → 7 days.
T3 → 1 day.
Effects of Thyroid Hormones (TH) on Metabolism
Impact on Lipid Metabolism:
Enhances fat mobilization, resulting in higher free fatty acid concentrations in the blood.
Stimulates fatty acid oxidation in various tissues.
Impact on Carbohydrate Metabolism:
Increases glucose uptake in an insulin-dependent manner.
Promotes both gluconeogenesis and glycogenolysis to elevate blood glucose levels.
Metabolic Rate Stimulation (except brain. spleen, testes):
Increases mitochondrial number and size as well as respiratory chain enzyme synthesis.
Upregulates Na⁺/K⁺ ATPase activity and affects membrane permeability, thereby influencing the metabolic rate.
Thyroid Hormones & Metabolic Regulation (BMR & REE)
15-40% of resting energy supports Na+/K+ ATPase activity.
Basal Metabolic Rate (BMR) & Resting Energy Expenditure (REE):
TH play a critical role as regulators of BMR—excess TH can elevate BMR by up to 100%, while TH deficiency may drop it by around 50%.
They enhance overall metabolic activity (increased oxygen consumption, ATP hydrolysis, and heat production).
TH can alter mitochondrial functions, including uncoupling oxidative phosphorylation and modifying mitochondrial hydrogen shuttles, thereby affecting the energy usage of major organs.
REE (energy for major organs) is highly TH-sensitive. (BMR & REE differ by ~10% and are often used interchangeably.)
Direct correlation between TH levels, energy expenditure, and body weight.
Hyperthyroidism (Hypermetabolic state) ↑ REE, weight loss, ↓ cholesterol, ↑ lipolysis & gluconeogenesis.
Hypothyroidism (Hypometabolic state) ↓ REE, weight gain, ↑ cholesterol, ↓ lipolysis & gluconeogenesis.
TH regulate metabolism via brain, white & brown adipose tissue, skeletal muscle, liver, pancreas.
Thyroid Disorders
Prevalence & Demographics:
Thyroid disorders affect approximately 3% of the population and are notably 9 times more common in women.
They can occur at any age.
Autoimmune Thyroid Diseases (AITD):
Over 95% of thyroid dysfunctions are autoimmune in nature, where antibodies either inhibit or stimulate thyroid hormone production.
Blocking Antibodies: Lead to reduced T4 production with a compensatory increase in TSH (hypothyroidism).
Stimulating Antibodies: Mimic TSH action, leading to thyroid overactivity (hyperthyroidism, e.g., thyrotoxicosis). Increased T4 production with a decrease in TSH
Central Hypothyroidism (hypothalamic/pituitary dysfunction):
Caused by TSH suppression due to pituitary tumors
TSH-secreting pituitary tumors exist but are rare
Clinical Cases of Hypothyroidism and Hyperthyroidism
Hypothyroidism - Hashimoto’s Thyroiditis
elevated TSH suggest primary thyroid dysfunction
thyroxine replacement therapy treatment
Hyperthyroidism - Graves’ Disease
TSH supression due to high circulating TH
antibodies bind TSH receptor, mimic TSH causing excess TH production
antithyroid drug carbimazole treatment
Consequences of iodine deficiency in pregnant women
Thyroid hormones play an essential role in neuronal migration, myelination, synaptic transmission during the early embryonic development of the nervous system.
The thyroid gland of the fetus does not produce thyroid hormones until approximately the 20th week of pregnancy.
Until then, it is dependent on maternal T4, which passes through the placenta into the fetus in very small amounts.
mental retardation of the fetus;
brain damage to both the fetus and young children in the first few years of life;
high infant mortality rate;
miscarriages and premature birth;
fetal growth retardation;
neonatal hypothyroidism;
Hormonal Control of Steroid Hormone Synthesis
Cholesterol is the precursor of all steroid hormones.
Pituitary hormones regulate steroid hormone synthesis:
LH (Luteinizing Hormone) → progesterone, testosterone
ACTH (Adrenocorticotropic Hormone) → cortisol
FSH (Follicle-Stimulating Hormone) → estradiol
Angiotensin II/III → aldosterone
The specific hormone synthesized depends on receptor expression in target cells.
Describe the Key Features and Functions of Steroid Hormones
Lipophilic (freely cross the membranes), derived from cholesterol (common precursor)
Produced upon stimulation and need, not stored or synthesised in advance
Synthesis enzymes in the mitochondrial + smooth ER
Require carrier proteins in blood due to water-insolubility:
CBG (corticosteroid-binding globulin) for cortisol
SHBG (sex hormone-binding globulin) for testosterone and estradiol
Metabolism in peripheral tissues modifies and modulates hormonal activity (esp. cortisol, not aldosterone)
Functional categories:
Glucocorticoids – carbohydrate metabolism e.g. Cortisol
Mineralocorticoids – mineral and electrolyte balance e.g. Aldosterone
Gonadocorticoids / Sex hormones – reproductive roles
Other Functions:
Inflammation control
Stress response
Mood, Cognition
Bone health and metabolism
describe Cholesterol Transport for Steroid Hormone Synthesis
Cholesterol Transport:
Cholesterol (from acetyl CoA via de novo synthesis or lipoproteins in blood)
Transported to mitochondria by StAR
Delivered to P450scc (inner membrane)
Converted to pregnenolone (first steroid intermediate)
Importance:
This is the initial and rate-limiting step in all steroid hormone biosynthesis.
Pregnenolone can then enter pathways to form:
Cortisol (glucocorticoid)
Aldosterone (mineralocorticoid)
Testosterone/estradiol (gonadocorticoids)
Key Proteins:
StAR (Steroidogenic Acute Regulatory Protein):
Location: Outer mitochondrial membrane
Function: Transfers cholesterol from the outer to inner mitochondrial membrane
Domain: Has a START domain (StAR-related lipid transfer domain)
Importance: Rate-limiting step in steroid hormone synthesis
Regulation: Stimulated by ACTH (via cAMP → PKA)
PBR (Peripheral Benzodiazepine Receptor) – now renamed TSPO (Translocator Protein):
Binds cholesterol and may assist in its import into mitochondria
Works with StAR in transporting cholesterol to the inner membrane
SBP (StAR-binding protein):
Less well characterized, possibly involved in stabilizing or transporting cholesterol
START family proteins:
A family of proteins related to lipid transfer (StAR is one of them)
Conversion of Cholesterol → Pregnenolone
Enzyme: P450scc (Cytochrome P450 Side Chain Cleavage enzyme)
Also called Desmolase or SCC enzyme
Encoded by: CYP11A1 gene
Location: Inner mitochondrial membrane
Reaction:
Cleaves the C20–C22 bond in cholesterol
Produces pregnenolone, the common precursor of all steroid hormones
Cofactors Required:
NADPH – provides electrons
Adrenodoxin and adrenodoxin reductase – electron carriers
Cytochrome P450 – hydroxylation before cleavage
Aldosterone - Function & Mechanism
Target organs: kidneys, salivary glands, colon
Targets ENaC (Epithelial Sodium Channel) → increases Na⁺ reabsorption, water retention, promotes K⁺ excretion
Regulates gene expression of ENaC → genomic effects, enhances mRNA production
Local aldosterone production also exists in some tissues e.g. cardiomyocytes, blood vessels, brain
Cortisol Peripheral Metabolism
11β-HSD Type 1
Location: Liver, adipose tissue, brain, skin
Function: Converts inactive cortisone → active cortisol (keto group at C11 to OH-group)
Net effect: Amplifies cortisol action in these tissues
11β-HSD Type 2
Location: Kidney, colon, salivary glands (aldosterone target tissues)
Function: Converts active cortisol → inactive cortisone (OH-group at C11 to a keto-group)
Purpose: Protects mineralocorticoid receptors (MR) from being activated by cortisol (which is abundant and has affinity for MR)
This allows aldosterone (which is more specific) to act effectively.
Without 11β-HSD type 2, cortisol would overstimulate mineralocorticoid receptors, leading to hypertension and hypokalemia — this happens in apparent mineralocorticoid excess (AME), a rare genetic disorder.
CRH (Corticotropin-Releasing Hormone) Role in Cortisol Release
Structure: 41 amino acid peptide
Source: Secreted by the paraventricular nucleus (PVN) of the hypothalamus
Function:
Stimulates the anterior pituitary to release ACTH
ACTH → stimulates adrenal cortex to produce cortisol
Signalling pathway: Activates cAMP second messenger system via Gs-coupled receptor
Example: During stress, CRH release increases → more ACTH → more cortisol → mobilizes glucose and suppresses immune responses.
Negative Feedback by Cortisol
ADH (Antidiuretic Hormone) Role in Cortisol Release
Vasopressin
Structure: 9 amino acid peptide
Source: Synthesized in supraoptic and paraventricular nuclei of the hypothalamus; stored and released from the posterior pituitary
Main roles:
Regulates water balance via kidneys
In HPA axis, acts synergistically with CRH to enhance ACTH release
Mechanism: Modulates intracellular calcium channels (via V1b receptors in the pituitary)
Example: In hypovolemia (low blood volume), ADH increases → helps conserve water and supports ACTH release → ↑ cortisol to maintain blood pressure and glucose.
Negative Feedback by Cortisol