L6-8 Cholinergic Drugs (Wang)
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
74 Terms
nervous system
processes regulated by autonomic nervous system (involuntary physiological processes)
heart rate
blood psi
respiration
digestion
sexual arousal
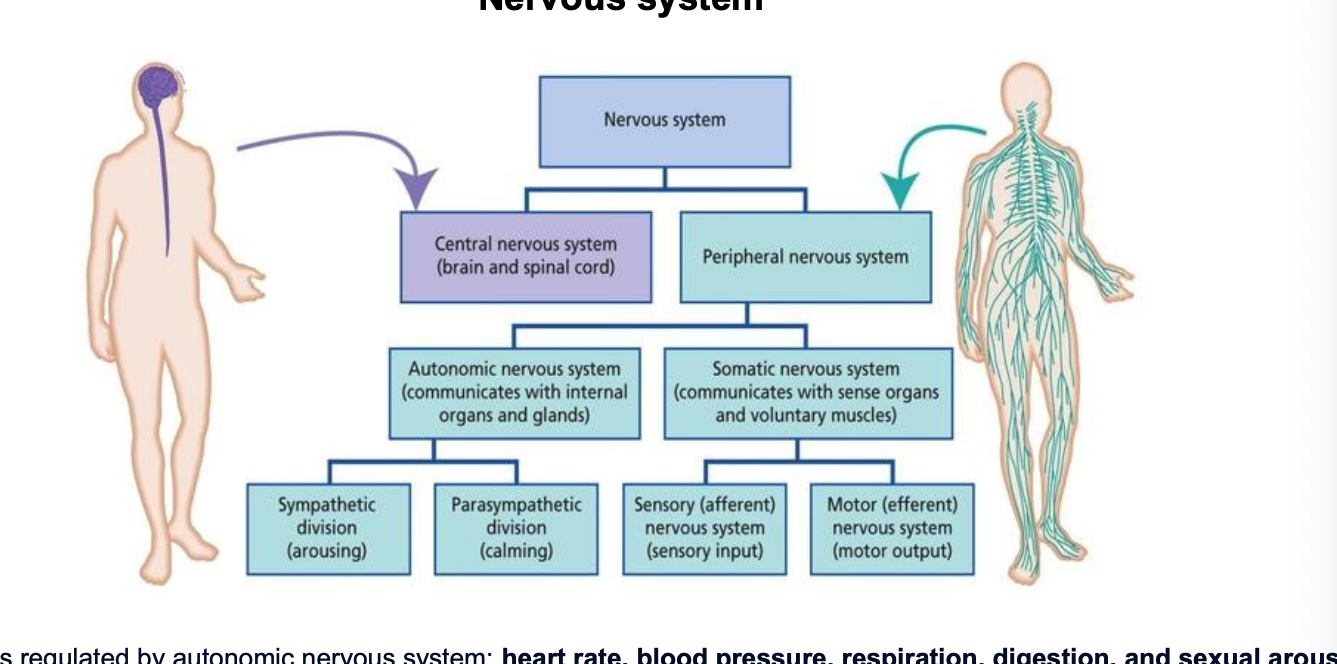
autonomic nervous system
autonomic nervous system controls specific body processes such as circulation of blood, digestion, breathing, urination, heartbeat, etc.
autonomic is named b/c it works autonomously i.e. withOUT a person’s conscious effort
parasympathetic → rest-and-digest
sympathetic → fight-or-flight
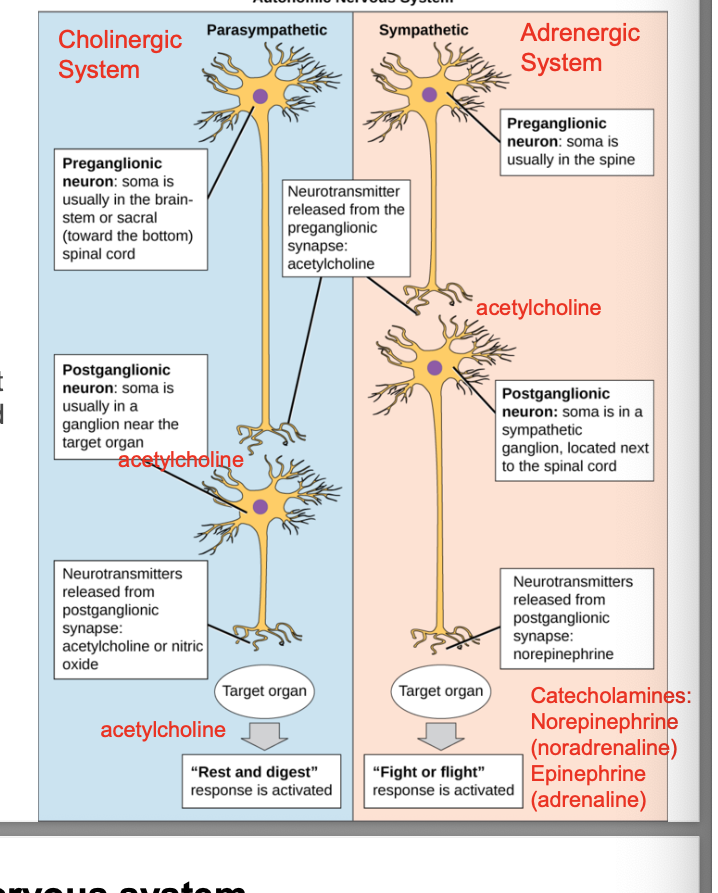
parasympathetic nervous system
eyes
constricts your pupil to limit how much light enters your eyes
also makes changes that can help improve close-up vision and cause tear production in the eyes
muscarinic agonist helps with glaucoma and induce miosis (constriction) in ocular surgery
nose and mouth
makes glands in mouth produce saliva and glands in nose produce mucus
can be helpful with digestion and breathing during times of rest
muscarinic agonist relieves the dry mouth of sjogren syndrome
lungs
tightens airway muscles and reduces the amount of work your lungs do during times of rest
muscarinic antagonist can help COPD and asthma
heart
lowers heart rate and pumping force of heart
digestive tract
increases rate of digestion and diverts energy to help digest food
also tells pancreas to make and release insulin → helps body break down sugars into a form your cells can use
muscarinic agonist can help ileus (cramping, sluggish gut)
waste removal
relaxes the muscles taht help you control when you pee or poop
muscarinic agonist can help with urinary retention (helps release piss); muscarinic antagonist can help with overactive bladder (keep pee in)
parasympathetic job = relax or reduce body’s activities
diseases you target parasympathetic system
myasthenia gravis → chronic autoimmune disorder that affects NMJ
dementia
cold smyptoms
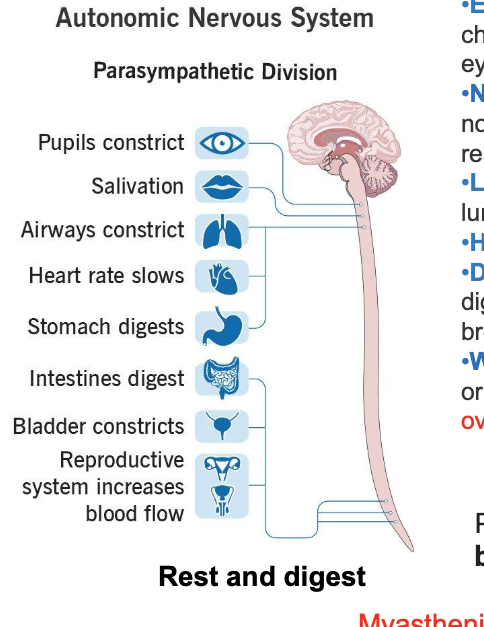
sympathetic nervous system
eyes
enlarge your pupils to let more light in and improve vision
heart
increase heart rate to improve delivery of oxygen to others parts of body
lungs
relax your airway muscles to improve oxygen delivery to your lungs
digestive tract
slow down digestion so its energy is diverted to other areas of body
liver
activate energy stores in liver to energy that can be used quickly
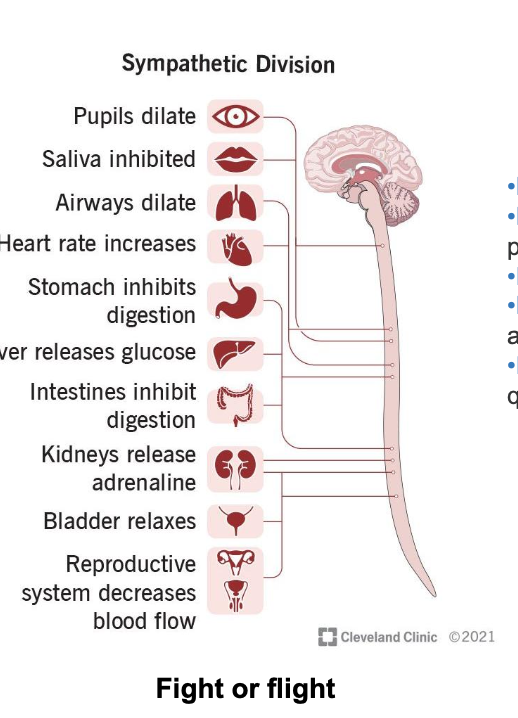
autonomic nervous system neuron transmitter and receptors
nerves of the ANS extend from CNS → smooth or cardiac muscles, organs and glands via a 2 neuron system; preganglionic and postganglionic
PNS
pre-synaptic neuron → acetylcholine → nicotinic receptor
post-synaptic neuron → acetylcholine → muscarinic receptors M1 - M5
SNS
pre-synaptic neuron → acetylcholine → nicotinic receptor
post-synaptic neuron → acetylcholine (muscarinic receptor), norepinephrine (adrenergic receptors α1, α2, β1, β2, β3)
nicotinic receptors are located on post-gangiolinic neurons of the sympathetic and parasympathetic cell bodies
respond to the binding of ACH which causes excitatory effect
muscarinic receptors located on ALL parasympathetic effector cells and some (sweat glands) sympathetic effector cells
respond to binding of ACH and have excitatory or inhibitory effect
adrenergic receptors located on most sympathetic effector cells
respond to the binding of NE which may have excitatory or inhibitory effect
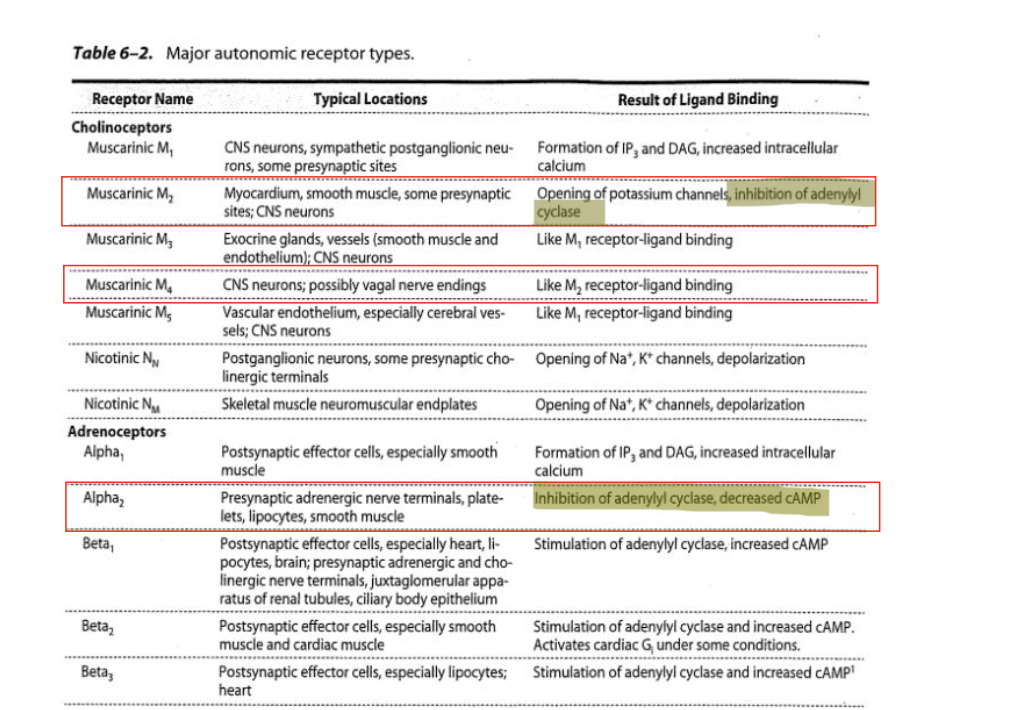
ANS receptors
muscarinic (M2)
locations
myocardium
smooth muscle
some presynaptic sites; CNS neurons
result of ligand binding
opening of K+ channels
inhibition of AC
muscarinic (M4)
location
CNS neurons; possibly vagal nerve endings
result of ligand binding
like M2 receptor-ligand binding
alpha (α2)
location
presynaptic adrenergic nerve terminals
platelets
lipoccytes
smooth muscle
result of ligand binding
inhibition of AC, decreased cAMP
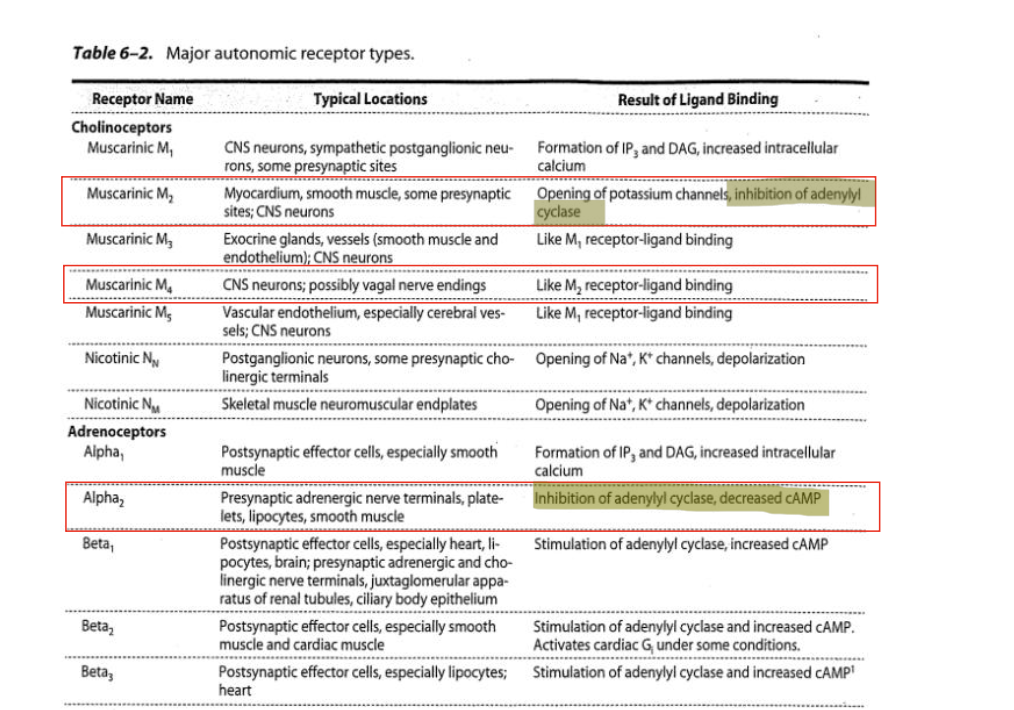
acetylcholine and cholinergic receptors
ACH is released by cholinergic neurons in the ANS and somatic NS and by some neurons in the CNS
cholinergic receptors are either muscarinic (mAChR) or nicotic (nAChR) based on their affinity for the naturally occurring alkaloids muscarine and nicotine, respectively
mAChR = receptor of relevance for cholinergic drugs targeting the GI and genitourinary (GU) systems
muscrainic receptors are GPCRs while nicotinic receptors are ligand gated ion channels
ACH is endogenous while muscarine and nicotine are exogenous
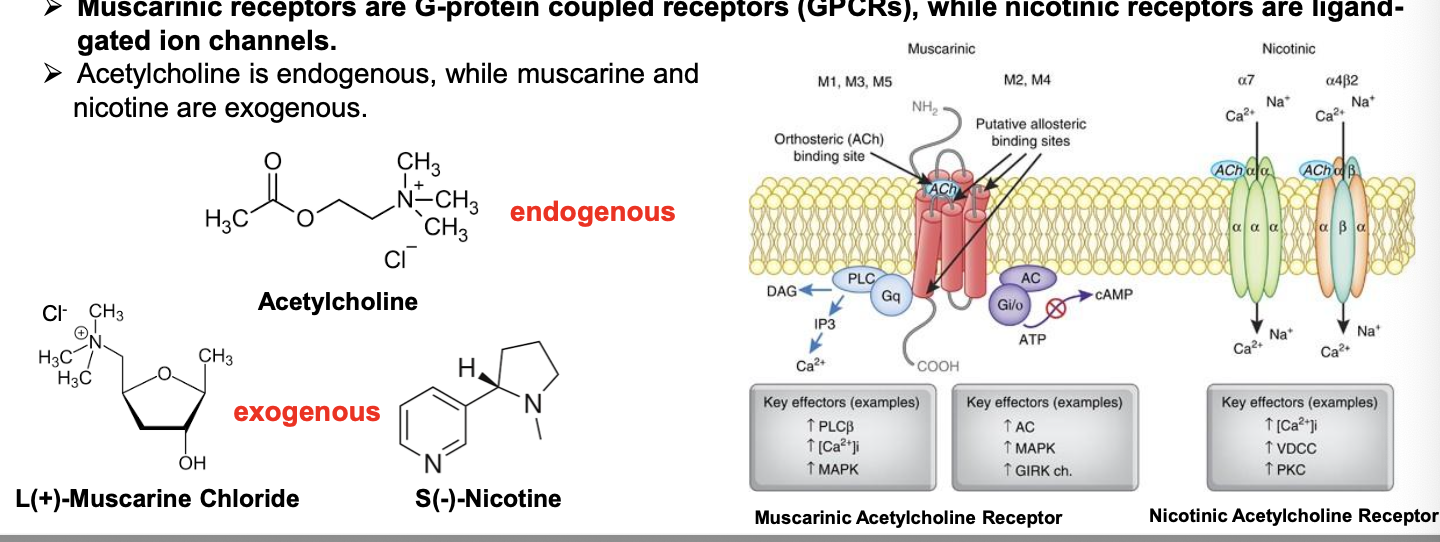
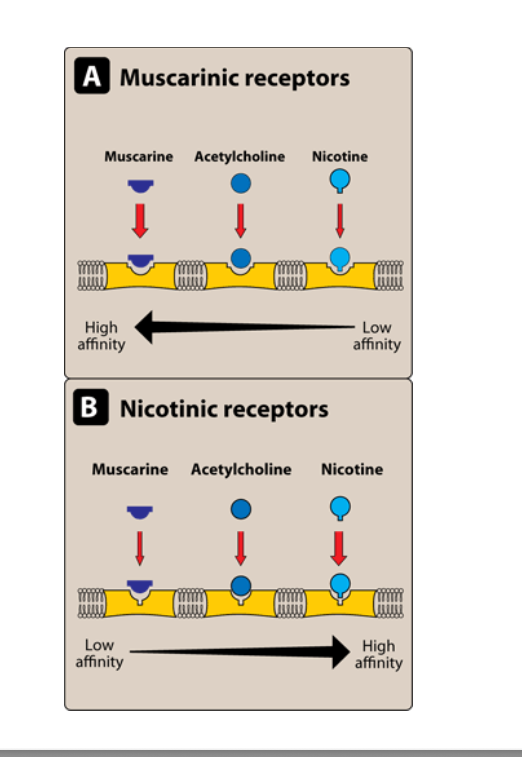
muscarinic vs nicotinic receptors
mucarinic receptors
low affinity to high affinity of substrates: nicotine → acetylcholine → muscarine
nicotinic receptors
low affinity to high affinity of substrates: muscarine → acetylcholine → nicotine
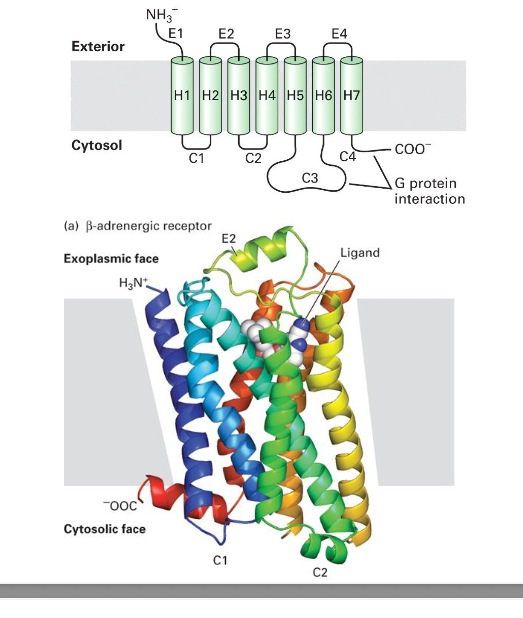
muscarinic acetylcholine receptor (GPCR family)
GPCRs, also known as seven-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor and G-protein linked receptors (GPLR) make up a large protein family of receptors that sense molecules outside the cell and activate inside signal transduction pathways and ultimately, cellular respones
called 7 transmembrane receptors b/c they pass thru cell membrane 7 times
basic structure
7 transmembrane domains (TMDs)
3 intracellular loops, 3 extracellular loops, N- and C- terminals
GPCRs = single-chain polypeptides consisting of approximately. 300 AA
high sequence conservation within the 7 transmembrane segment core; more diversity within the cytoplasmic loops and C terminus
naming a few GPCRs
adrenergic receptor
muscarinic receptor
dopamine receptor
histamine receptor
opioid receptor
serotonin receptor
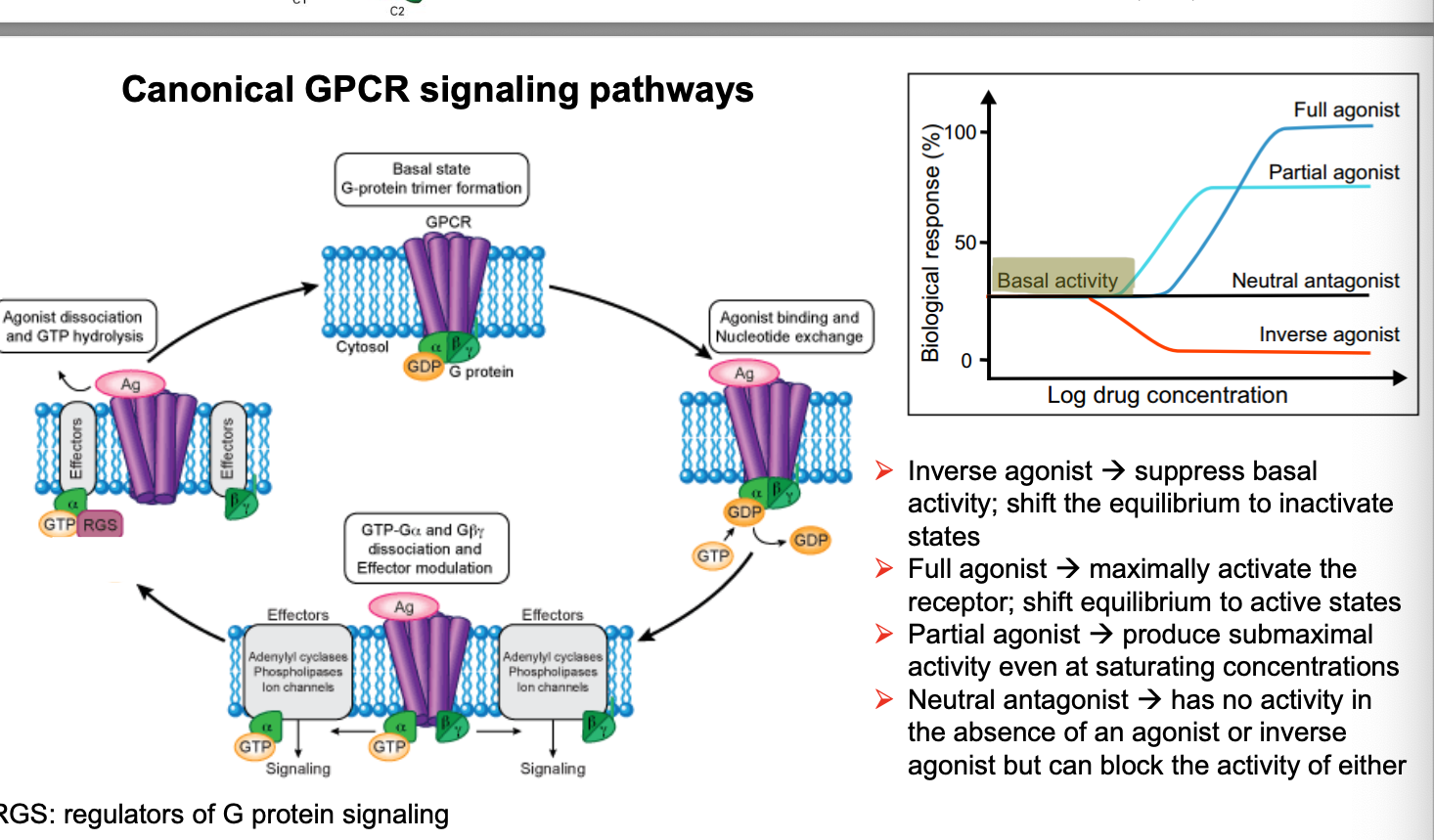
canonical GPCR signaling pathways
inverse agonist → suppress basal activity; shift the equilibrium to inactivate states
full agonist → max. activate the receptor; shift equilibrium to active states
partial agonist → produce submaximal activity even at saturating concentrations
neutral antagonist → has NO activity in the absence of an agonist or inverse agonist but can block the activity of either
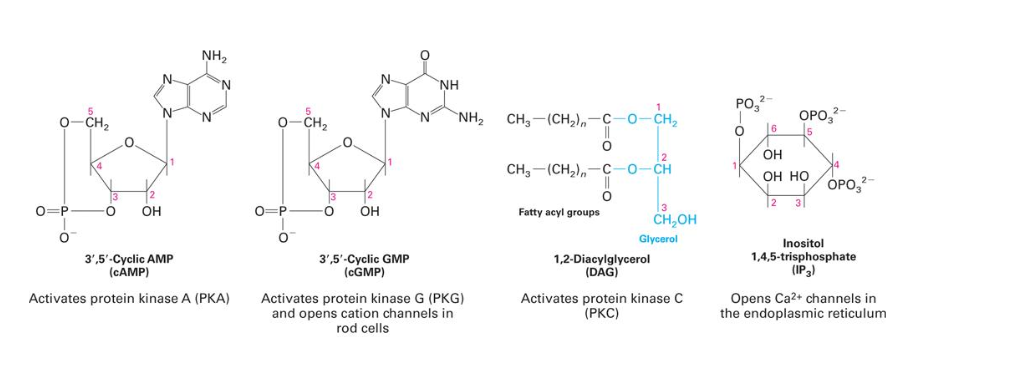
second messengers
2nd messengers = molecules that relay signals received at receptors on the cell surface - such as arrival of protein hormones, growth factors, etc. - to target molecules in the cytosol and/or nucleus
3 major classes of 2nd messengers
cyclic nucleotides (cAMP and cGMP) → activate PKA and PKG
inositol triphosphate (IP3) and diacylglycerol (DAG) → products of PIP2 by PLC; IP3 activates ligand-gated Ca2+ channel in ER; DAG activates PKC
calcium ions (Ca2+); cytosol 0.1 µM, extracellular 1 mM → bind to and activate calmodulin → modulate Ca2+/calmodulin dependent protein kinases
GPCR signaling mediated by PLC (α1 adrenergic receptor, Gq)
signal molecule binds to GPCR
G protein is activated by exchanging GDP for GTP
G protein activates PLC
PLC cleaves PIP2 into DAG and IP3
IP3 difuses to the ER where it binds to IP3-gated Ca2+ channels
Ca2+ ions are released into the cytoplasm
Ca2+ ions bind to calmodulin forming a complex that activates various proteins → cellular responses
signal molecule can bind to tyrosine kinase receptor that activates PLC → PIP2 blah blah blah
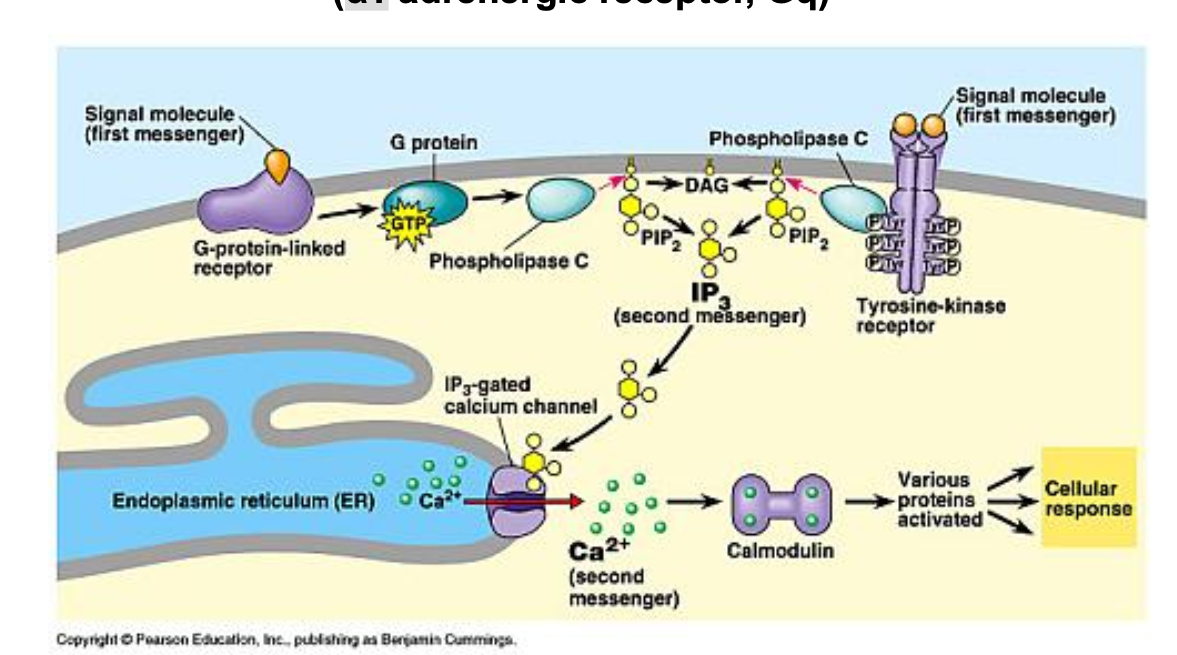
GPCR signaling mediated by cAMP (β adrenergic receptor, Gs)
odorant molecule binds to G protein
G protein is activated by exchanging GDP for GTP
G protein stimulates AC which converts ATP into cAMP
cAMP activates cyclic nucleotide-gated Na+/Ca2+ channel, allowing Na+ and Ca2+ ions to enter the cell
influx of Ca2+ leads to additional signaling events including activation of Ca2+gated Cl- channels which results in Cl- efflux, further depolarizing thee cell
some Ca2+ binds to calmodulin (CAM) → modulating additional signaling pathways and facilitating ion exchange thru Na+/Ca2+ exchanger
GPCR can regulate ion channels thru either direct interaction or 2nd messenger-mediated mechanism
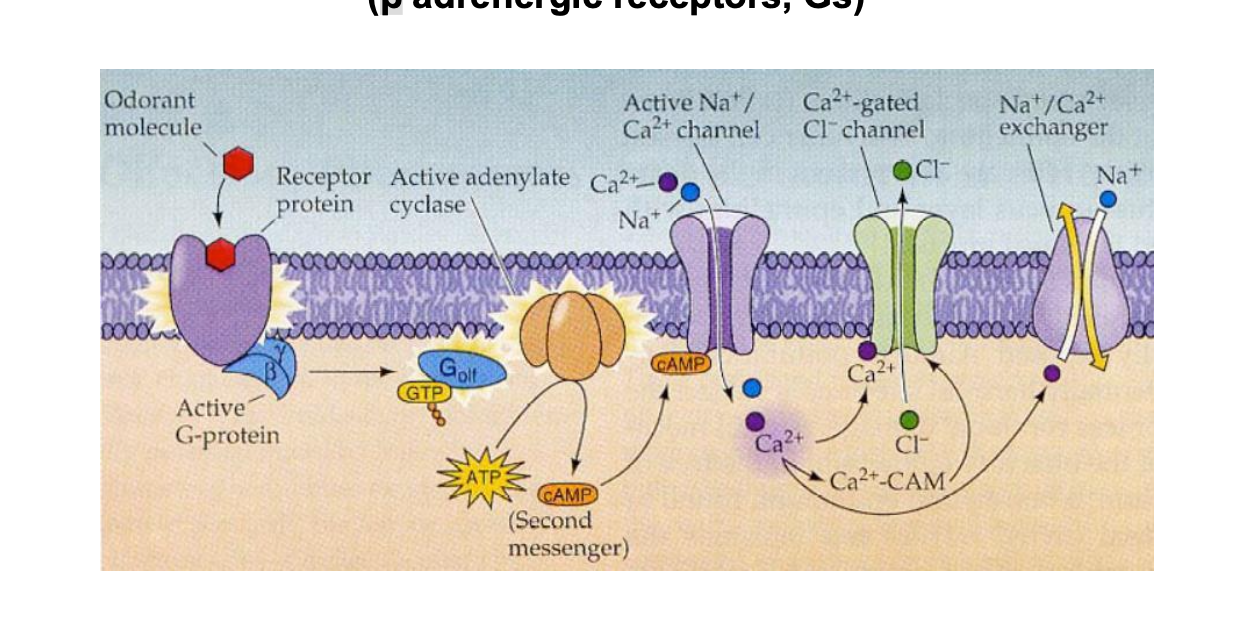
nicotinic acetylcholine receptor
2 binding sites for the endogenous ligand ACH exist on the extracellular domain of the nAChR molecule
1 binding site is located on each α subunit at the αγ and αδ interfaces.
the binding sites show a positive cooperativity meaning that ligand binding to 1 site facilitates binding to the other
each of the 5 transmembrane subunits is composed of 4 hydrophobic membrane spanning segments (M1-M4)
ligand binding induces a conformation change in the receptoor → opening ion channel and allowing passage of Na+ and K+ thru the center of the protein → result = depolarization of surrounding plasma membrane
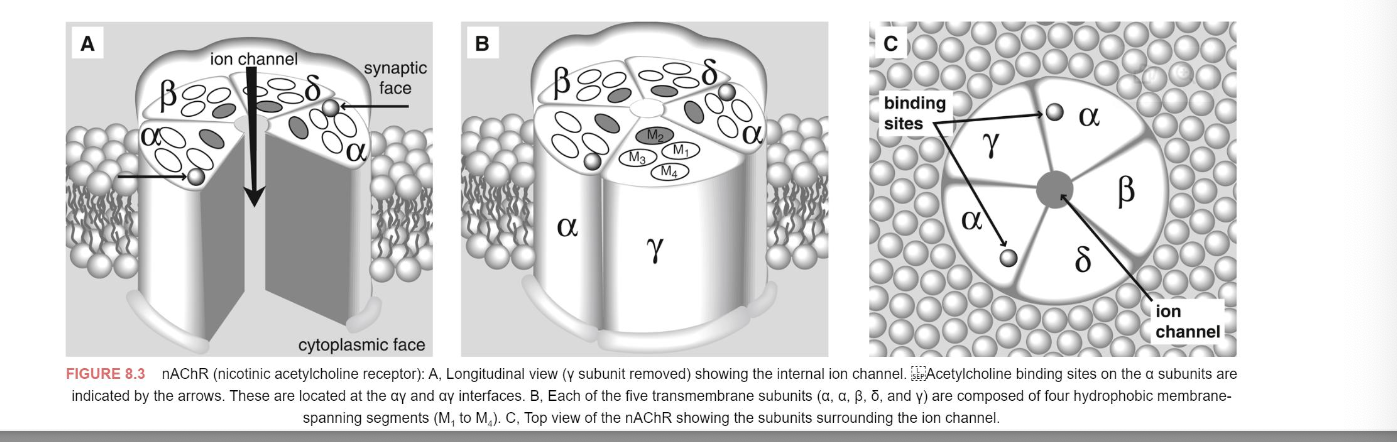
cholinergic drugs (direct or indirect agonists)
parasympathomimetic drug also called cholinomimetic drug or cholinergic receptor stimulating agent is a substance that stimulates the parasympathetic nervous system
common uses of parasympathomimetic include glaucoma and underactive bladder
muscarinic or nicotinic agonists and acetylcholine esterase inhibits produce similar biological effects
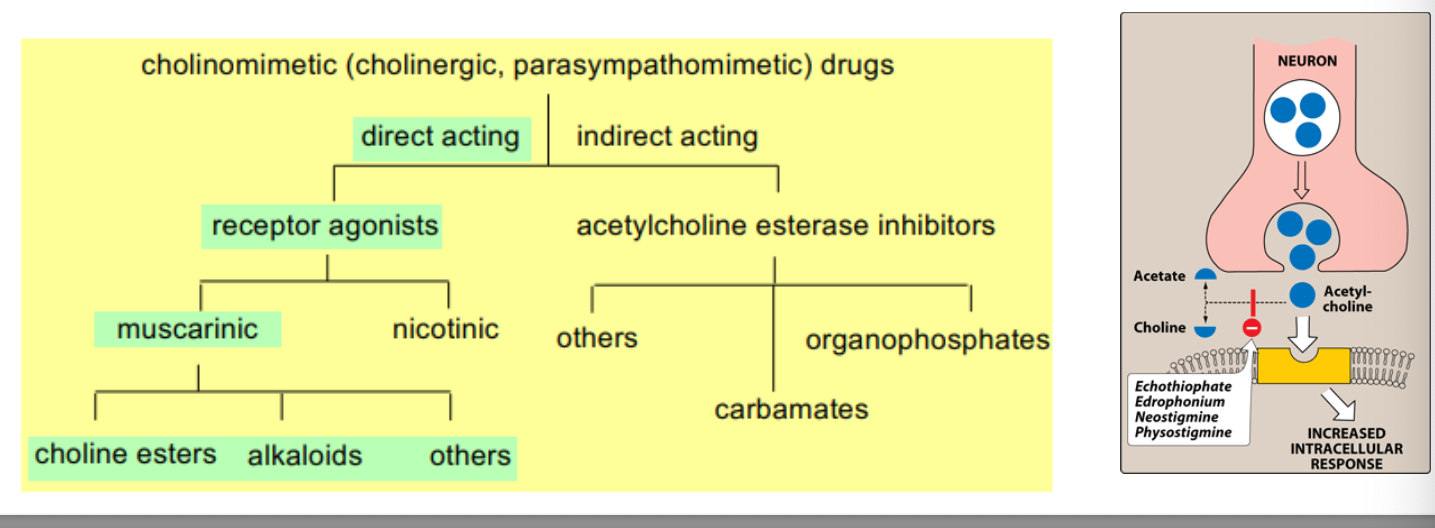
acetylcholine biosynthesis and hydrolysis
neurotransmisison in cholinergic neurons involve 6 sequential steps
synthesis of ACH
choline + AcCoA → ACH
transport of choine is inhibited by hemicholinium
uptake into storage vesicles
ACH is protected form degradation in the vesicle
release of neurotransmitter
release is blocked by botulinum toxin
spider venom causes release of ACH
binding to the receptor
post-synaptic receptor is activated by binding of neurotransmitter
degradation of acetylcholine
ACH is rapidly hydrolyzed by acetyl-cholinesterase in the synaptic cleft
recycling of choline
choline is taken up by neuron
serine → acetylcholine pathway
serine → ethanolamine
thru serine decarboxylase
ethanolime → choline
thru choline N-methyl-transferase and SAM (gives methyl group?)
choline → acetylcholine
thru acetyl S-CoA and choline acetyltransferase (ChAT)
acetylcholine degradation/recycling pathway
acetylcholine → acetate and choline
thru H2O, AChE
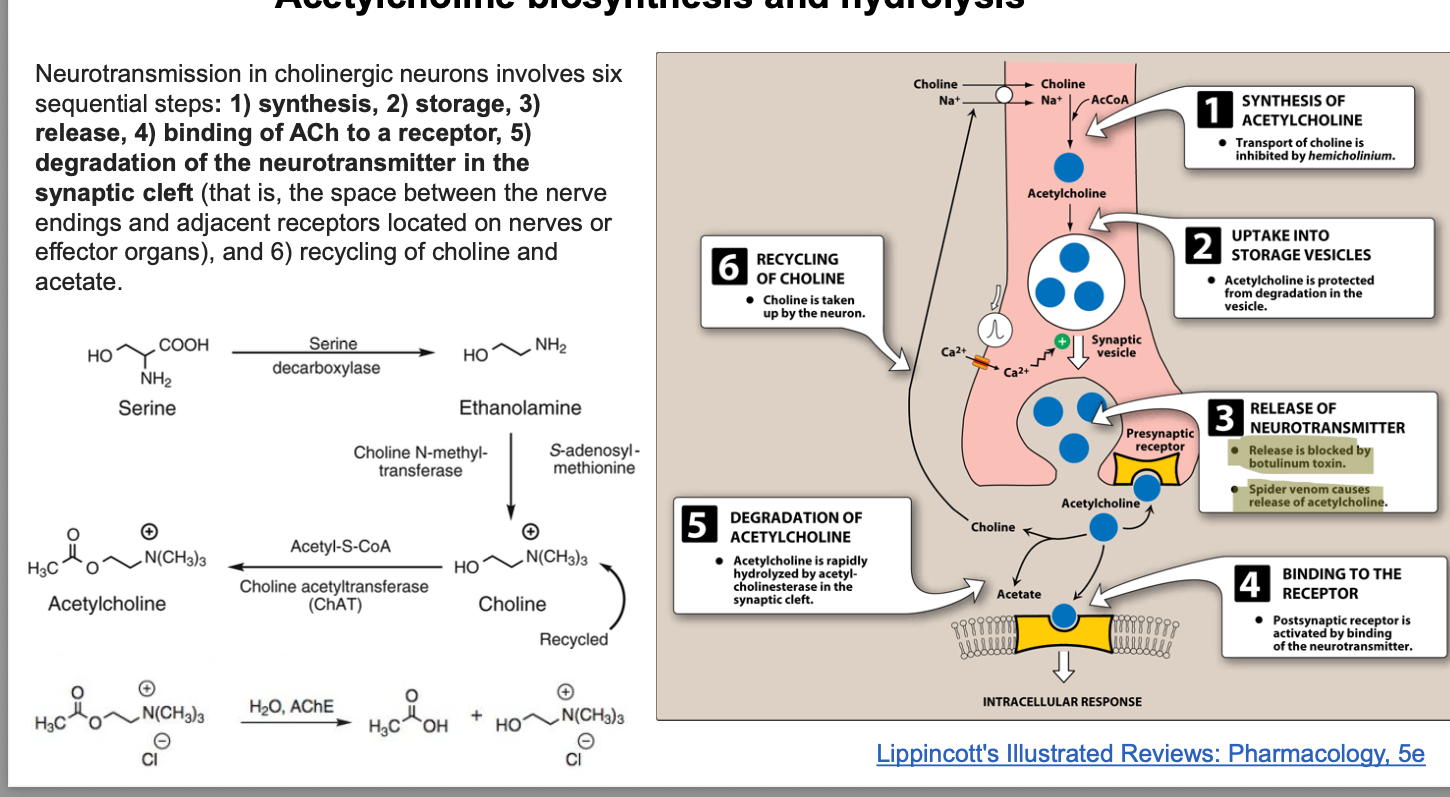
botulinum toxin (botox) inhibits the release of ACH
botulinum toxin = widely known for causing a type of food poisoning known as botulism
botulinum toxin (BTX or BonT) = protein produced by spore-forming, anaerobic, bacilli bacterium clsotridium botulinum
botulinum toxins work by inhibiting the release of the ubiquitous neurotransmitter ACH → paralyzing muscle
botox = cosmetic uses; mainly used to relax the muscles that cause wrinkles
pathway
botulinum toxin endocytosed into presynaptic nerve terminal at NMJ → light portino of toxin is released into the cytosol → cleaves specific SNARE proteins
type B, D, F, G → cleave synaptobrevin
type A, C, E → cleave SNAP-25
type C → cleaves syntaxin
→ cleavage of SNARE proteins prevents the formation of SNARE complex which is essential for fusion of synaptic vesicles w/pre-synaptic membrane → ACH NOT released into synaptic cleft
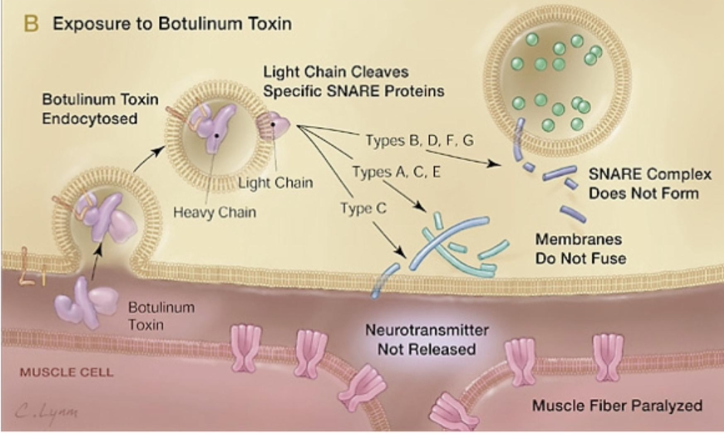
α-latrotoxin promotes the release of ACH
α-latrotoxin = pre-synaptic neurotoxin isolated from the venom of the black widow spider Latrodectus tredecimguttatus
exerts toxic effects in the vertebrate CNS by depolarizing neurons → increases concentration of Ca2+ and by stimulating uncontrolled exocytosis of neurotransmitter from nerve terminals
diagram
α-latrotoxin binds to pre-synaptic protein (neurexin) → stimulates massive Ca2+ influx → increased Ca2+ triggers vesicles with neutrotransmitter to fuse with pre-synaptic membrane and release → neurotransmitter bind to ligand-gated Na+ channels → Na+ influx into post-synaptic cell generates new electrical signal
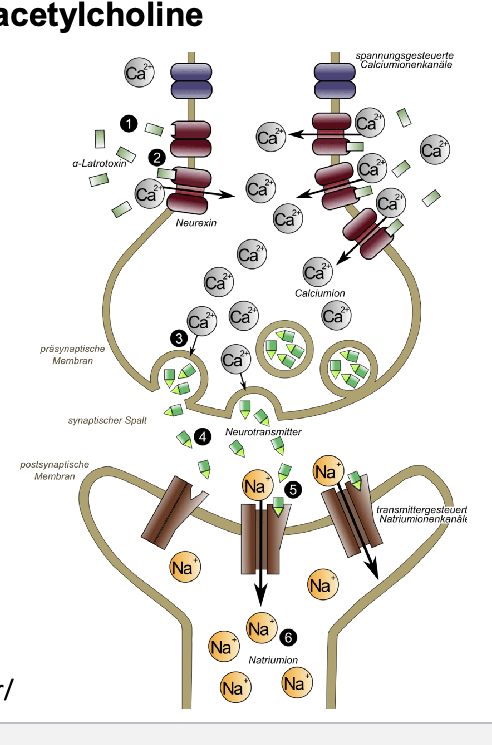
α-Bungarotoxin inhibits nicotinic acetylcholine receptors
α-Bungarotoxin (found in cobra venom) = nicotinic receptor antagonist that binds irreversibly to the receptor → inhibiting the action of ACH at the NMJ
binding = highly specific/irreversible → leads to muscular weakness, paralysis, respiratory failure → paralysis can lead to death
in the CNS and PNS, α-Bungarotoxin acts by inducing paralysis in skeletal muscles by binding to subtype of nicotinic receptors α7
muscarinic acetylcholine receptors (mAChR)
5 subtypes of receptor designated M1-5
odd numbered receptors (M1,M3,M5) = coupled to Gq/G11 class
activates PLC to hydrolyze phosphtatidylinositol 4,5-diphosphate → DAg and IP3 as intracellular messengers
even numbered receptors (M2, M4) = coupled to Gi/Go class
mediates inhibiton of AC
left diagram
esteric site is responsible for binding with ester bond of ACH (hydrogen bond)
anionic site contains negatively charged AA that interact with positively charged quaternary ammonium group of ACH
right diagram
even numbered muscarinic receptors
ACH binds to receptor → activates Gi protein → activated Gi protein inhibits AC → decreases conversion of ATP to cAMP → lowered cAMP levels lead to reduced activation of PKA → decreased phosphorylation of target proteins
odd numbered muscarinic receptors
ACH binds to receptor → activates Gq → Gq activates PLC → PLC hydrolyzes PIP2 into DAG and IP3 → DAG activates PKC while IP3 increases intracellular Ca2+ → cellular responses
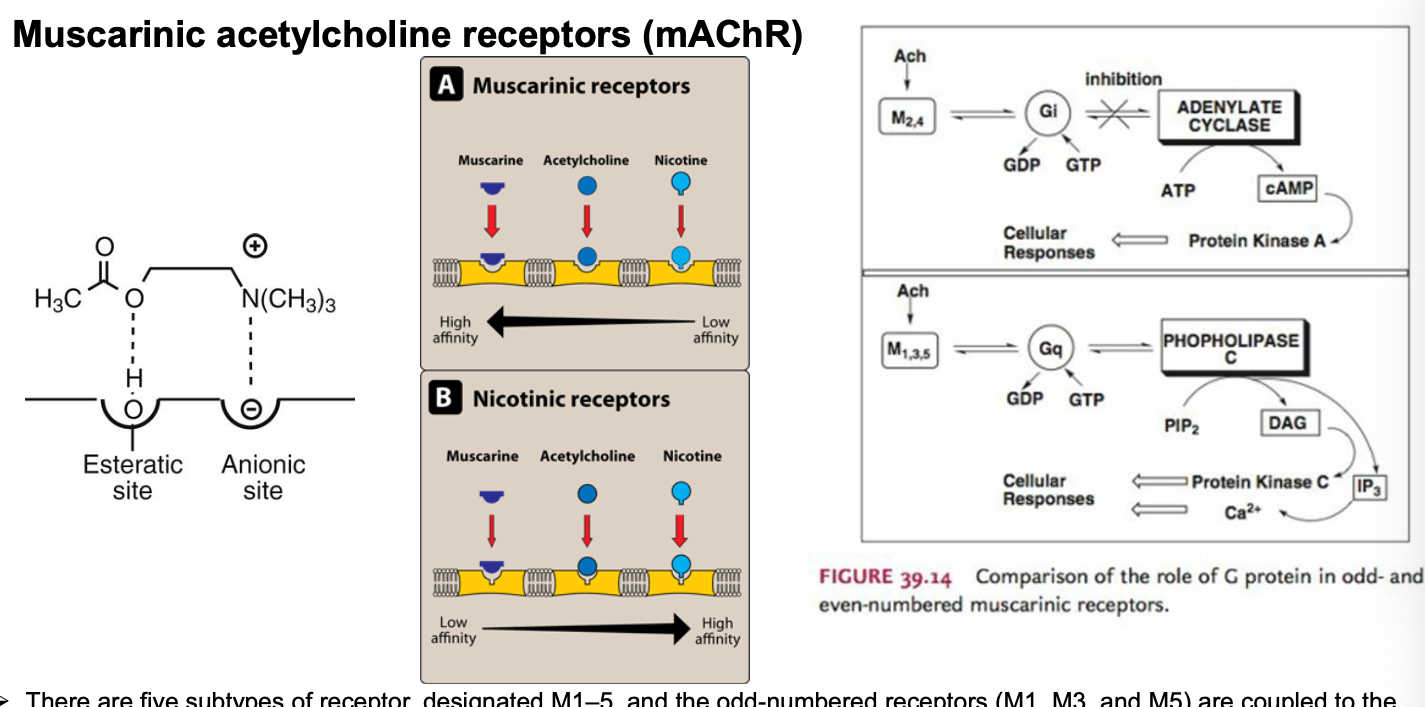
muscarinic receptor agonists
muscarine = 1st parasympathomimetic substance ever studied and causes profound parasympathetic activation that may end in convulsions and death
muscarine mimics the function of the natural neurotransmitter ACH in the muscarinic part of thecholinergic nervous system (non-selective agonist of muscarinic ACH receptors)
clinical signs of cholinergic overstimulation usually develop between 0.5-2 hours after ingestion; lethal dose of muscarine = 40 mg - 495 mg
specific antidone = atropine
alkaloid and inhibits ACH and muscarine by binding to muscarinic receptors (antagonist)
alkloid = class of nitrogenous organic compounds of plant origin which have pronounced physiological actions on humans
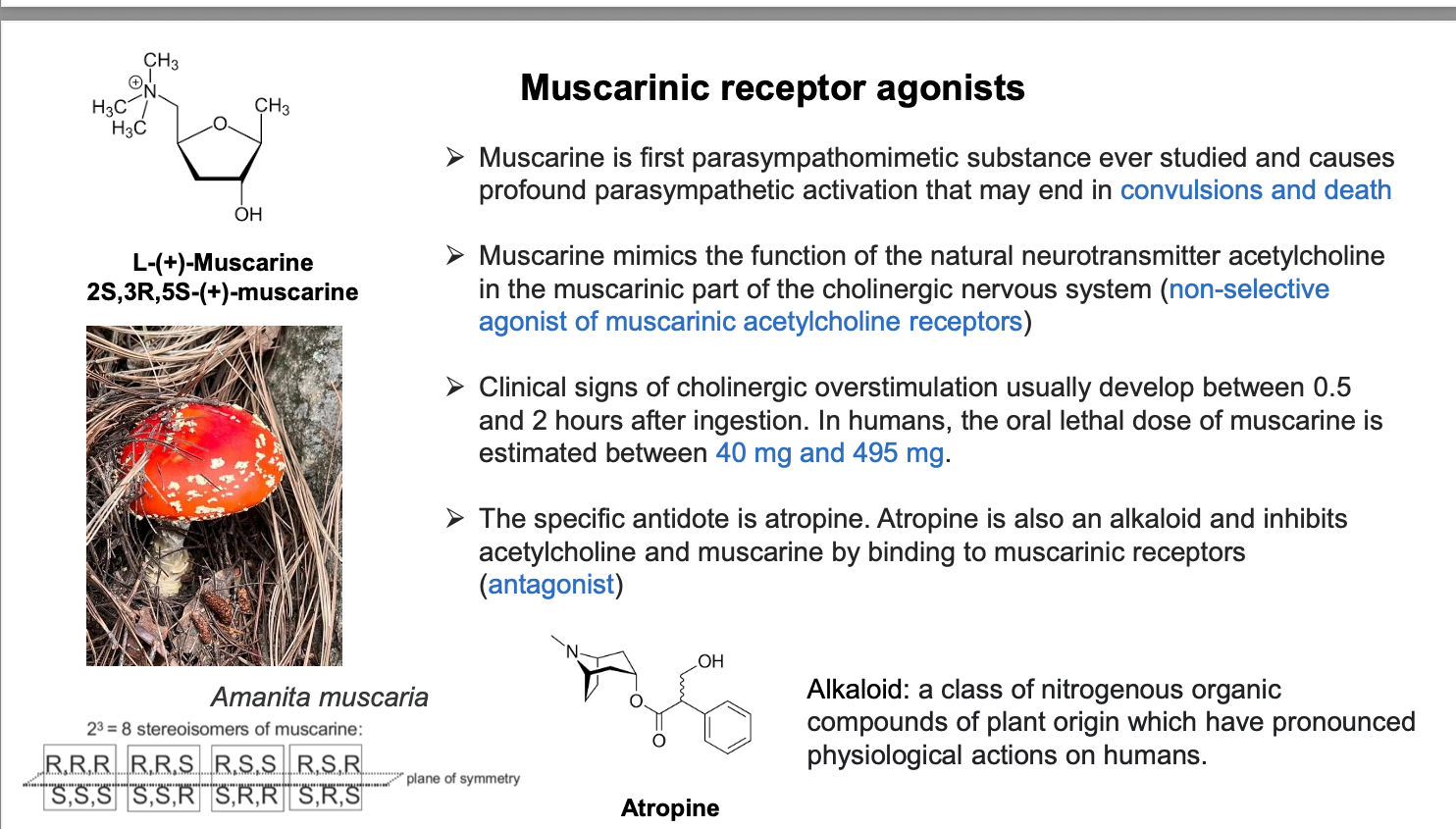
stereochemistry pt 1
at first, enantiomers were distinguished by their ability to rotate the plane of polarize light. isomers that rotate the plane of polarized light to the right, clcokwise, designated as dextrorotatory, indicated by + sign before chemical name. the oppo- site designation, levorotatory, or (-) was assigned to molecs that rotate the plane of polarized light to the left or in counterclockwise direction
D, L (+), (-) CANNOT be determined by chemical structure
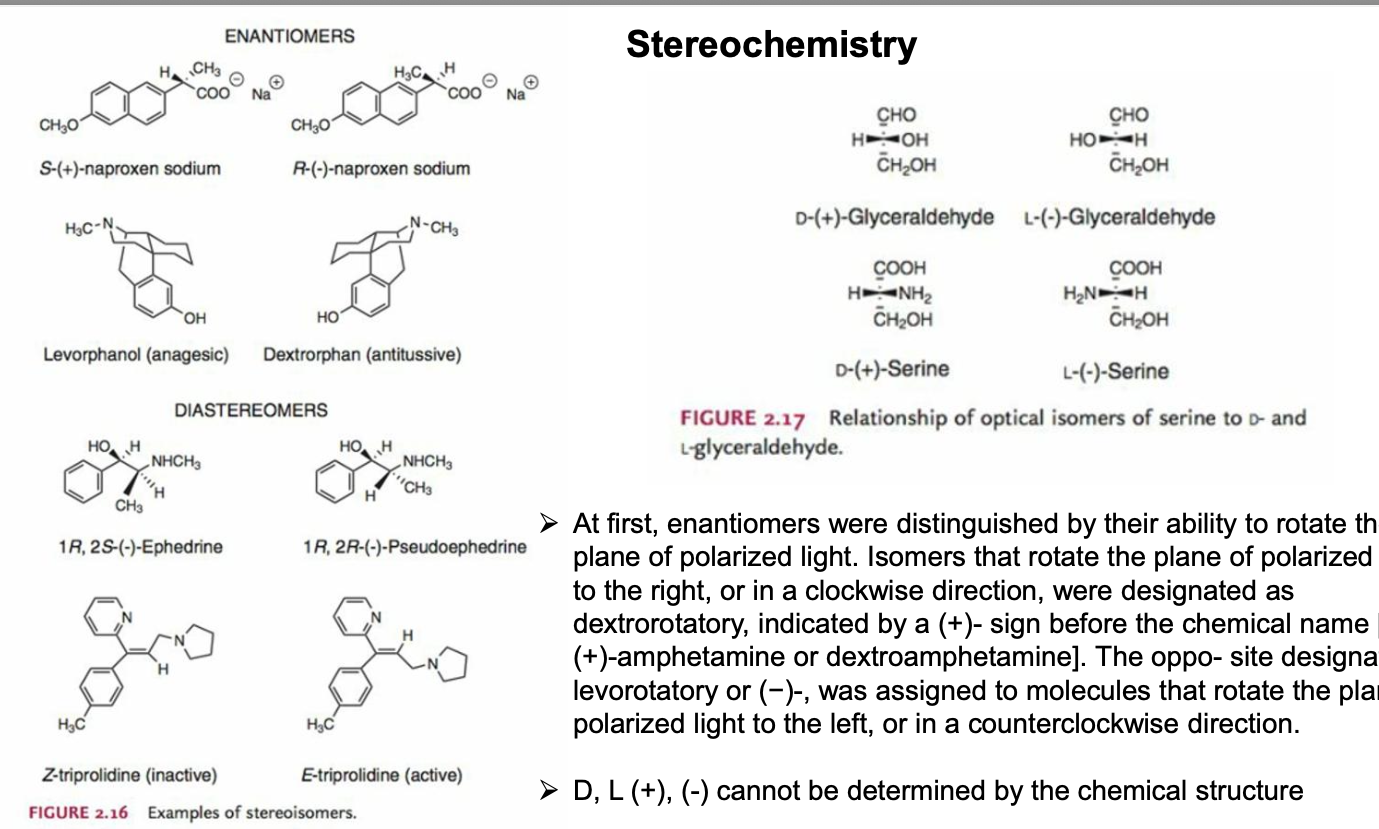
stereochemistry pt 2
enantiomers = mirror image pairs with opposite configuration at both stereocenters and identical connectivity
diastereomers = differ at one stereocenter; have at least one identical stereocenter; NOT mirror images
stereochemistry pt 3
sequence rule for assignment of config to chiral centers
the higher the atomic # of the immediate substituent atom → higher the priority. for example H < C < N < O < Cl (different isotopes of the same element are assigned a priority according to their atomic mass)
if 2 substituents have the same immediate substituent atom, evaluate atoms progressively further away from the chiral center until a difference is found. for example, CH3 < C2H5 < ClCH2 < BrCH2 < CH3O
if double or triple bonded groups are encountered as subtitutents, they are treated as an equivalent set of single-bonded atoms. for example: C2H5 < CH2=CH < HC☰C
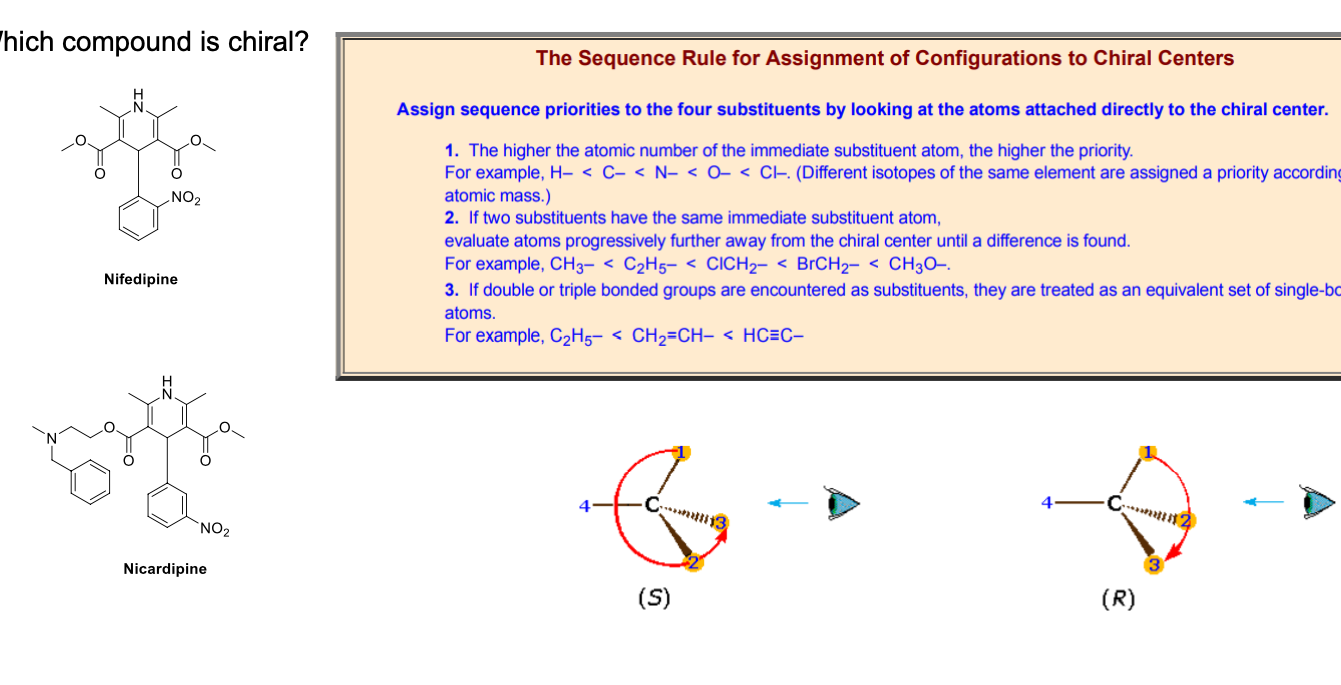
diagram
nicardipine is chiral; nifedipine is achiral
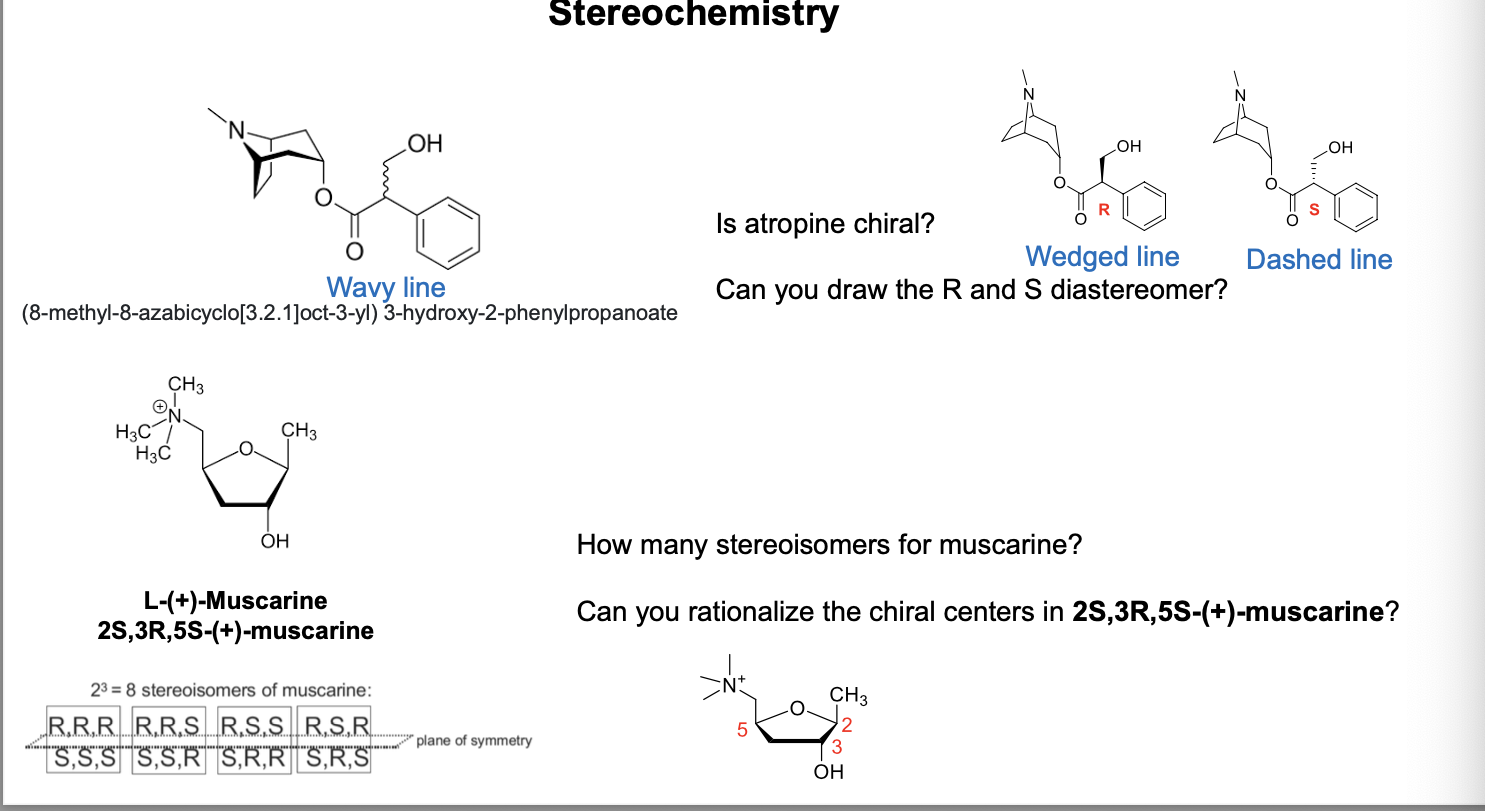
stereochemistry pt 4
atropine is chiral
wavy bond at benzylic OH indicates unspecified configuation; can be R or S; used when stereocenter’s configuration is unknown or a mixture is present
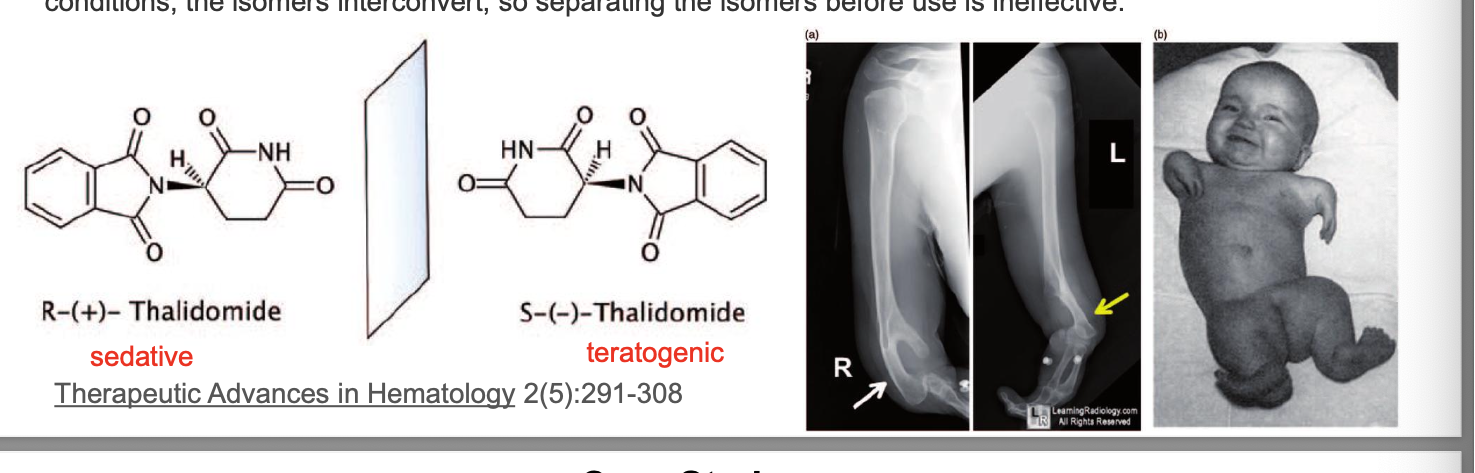
why does stereochemistry matter?
thalidomide = tragic history; introduced in germany in 1957 as a sedative and hypnotic and was marketed OTC largely as a drug for treating morning sickness in preggo women
in the following few years, 10k infants were born with phocomelia or limb malformation; only half of the infants survived, some of those who did have other defects in addition to limb deficiences
thalidomide exists in 2 mirror-image forms; racemic mixture of R and S-enantiomers; R enantiomer → sedative effects; S isomer = teratogenic; under biological conditions the isomers interconvert so separating the isomers BEFORE use is ineffective

case study
a 56 year old man reports to the ER after eating mushrooms he has foraged. he has brought some fo the whole, uncooked mushrooms with him.
a) although his symptoms now, 1 hour after ingesting the mushrooms, only include mild abdominal pain and iarrhea, what symptoms should one look for?
b) the mushrooms he has with him has been IDed as amanita muscaria. what is the treatment for this type of mushroom poisoning?
a) must ID the specific mushroom. amanita muscaria mushrooms contain muscarine, a potent muscarinic receptor agonist, and symptoms will be related to the stimulation of muscarinic receptors (convulsions and death); mushrooms of the psilocybe or panaeolus species contain psilocybin which will cause short-lasting hallucinations; mushrooms of the amanita phalloides or related species contain amatoxins which can cause hepatic and renal failure and ultimately death
b) treatment with atropine effectively blocks the effects of muscarinic receptor stimulation; large doses may be required. if the pt is showing signs of CNS excitations and hallucinations, these symptoms should be treated with a benzodiazepine. atropine often exacerbates the delirium.
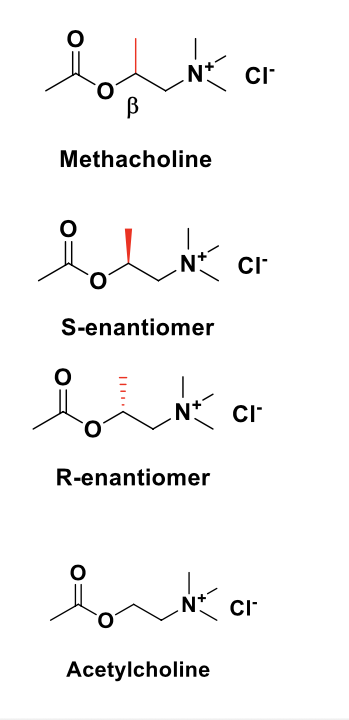
muscarinic agonists (direct cholinomimetics) - methacholine
methacholine = marketed as enantiomeric mixture
selective muscarinic agonist; little activity at nAChRs
S-enantiomer = 240 fold more potent than R-enantiomer at mAChRs
S-enantiomer = more resistant to AChE hydrolysis than ACH (54%) while R-enantiomer is a weak inhibitor of AChE
formulated for inhalation and used for the diagnosis of asthma
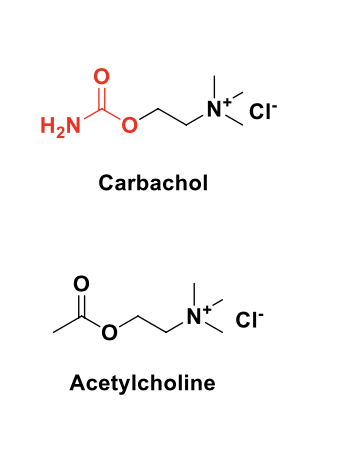
muscarinic agonists (direct cholinomimetics) - carbachol
carbachol is a carbamate of ACH
more resistant towards acid-, base-, or AChE-catalyzed hydrolysis than ACH
agonist of both mAChRs and nAChRs (non-selective)
used for the treatment of glaucoma and induction of miosis (constriction) in ocular surgery)
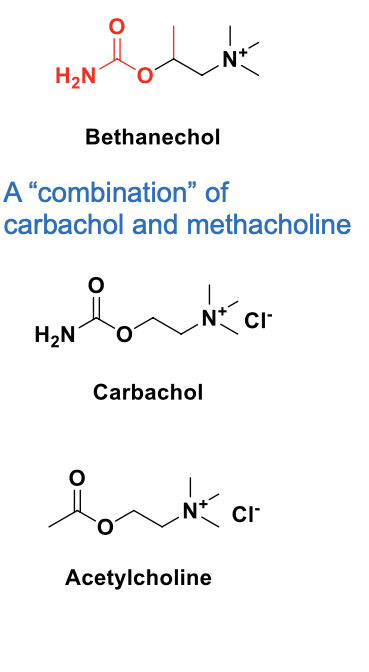
muscarinic agonists (direct cholinomimetics) - bethanechol
bethanechol = selective agonist for mACHRs (similar to methacholine)
NOT hydrolyzed by AChE; long duration of action
given orally for the treatment of ileus (partial or complete non-mechanical blockage ) and urinary retention which are side effects of general anesthetic
a combo of carbachol and methacholine
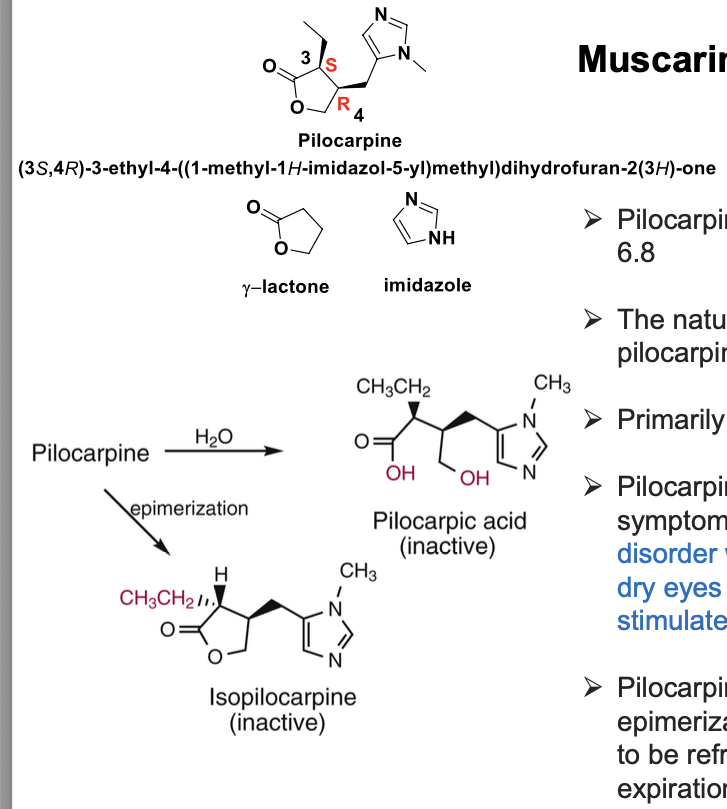
muscarinic agonists - pilocarpine
pilocarpine is a natural alkaloid with pKa = 6.8
naturally occuring alkaloid = 3S, 4R-(+)-pilocarpine
primarily acts on M3 mAChR
used in the treatment of symptoms of sjogren syndrome (immune disorder which destroys glands, resulting in dry eyes and dry mouth); pilocarpine can stimulate the secretion of saliva and tears
pilocarpine = prone to hydrlysis and epimerization and the gel formulation needs to be refrigerated and labeled with a 2 week expiration date
pKa
pKa = pH + log [acid]/[base]
pH < pKa
protonated forms HA and BH+ pre-dominante
pH = pKa
[HA] = [A-] and [BH+] = [B]
pH > pKa
deprotonated forms [A-] and [B] predominate
conjugate base = deprotonated form of acid
![<ul><li><p>pK<sub>a</sub> = pH + log [acid]/[base]</p></li><li><p>pH < pK<sub>a</sub></p><ul><li><p>protonated forms HA and BH+ pre-dominante</p></li></ul></li><li><p>pH = pK<sub>a</sub></p><ul><li><p>[HA] = [A-] and [BH+] = [B]</p></li></ul></li><li><p>pH > pK<sub>a</sub></p><ul><li><p>deprotonated forms [A-] and [B] predominate</p></li></ul></li><li><p>conjugate base = deprotonated form of acid</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/4d47b3ea-0747-43bc-b4a9-20ed9d969ae0.png)
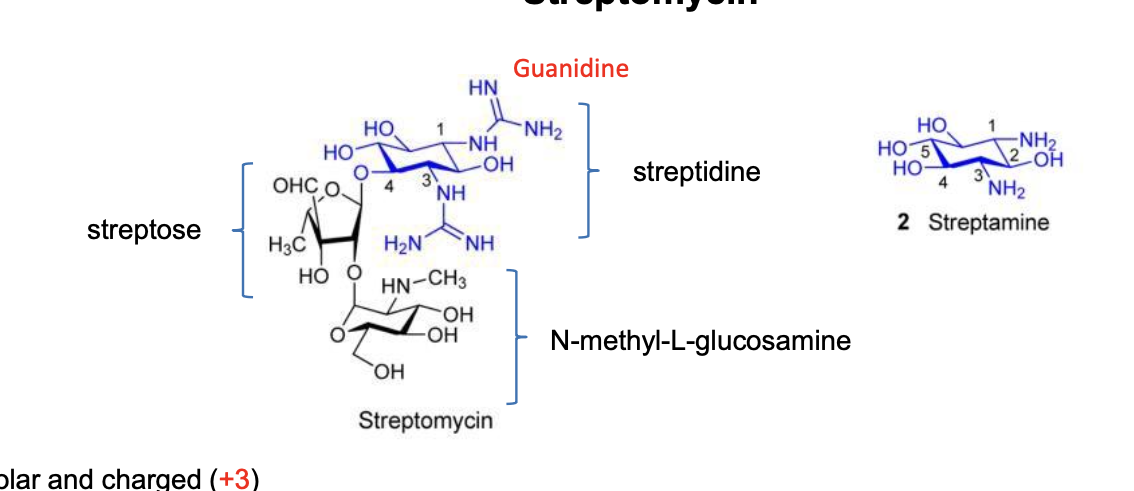
streptomycin
polar and charged +3
have to be injected IM or IV
CANNOT cross the BBB (NOT effective for meningitis)
entry mechanisms
binds to negatively charged groups on the outer surface of the cell membrane which displaces Mg2+ and Ca2+, creating pores thru which aminoglycosides can pass
streptomycin was the 1st effective agent used against TB
use is limited by ototoxicity, nephrotoxicity, and drug resistance
structure
made of streptose
N-methyl-L-glucosamine
streptidine
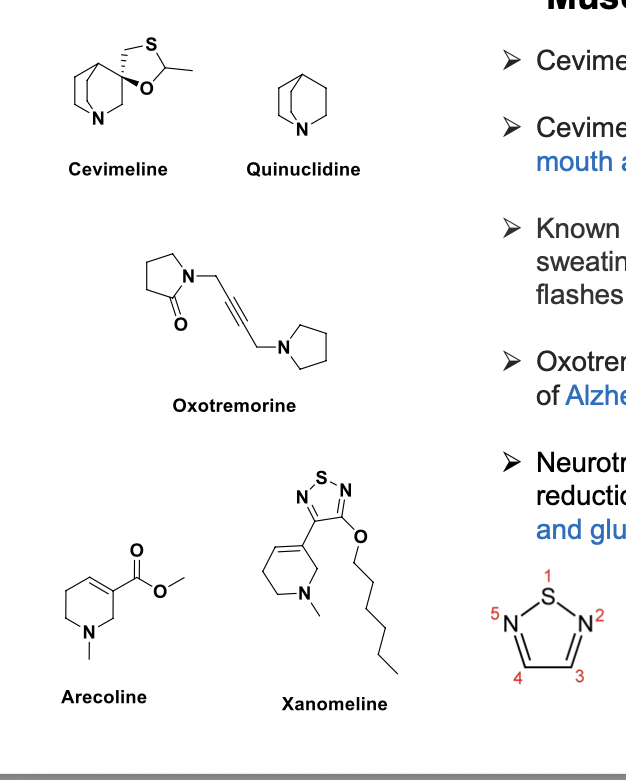
muscarinic agonists - cevimeline and others
cevimeline = quinuclidine analog and is a mAChR agonist
available as oral capsule for treatment of dry mouth associated with sjogren syndrome (similar to pilocarpine)
known side effects
nausea
vomiting
diarrhea
excessive sweating
rash
headache
runny nose
cough
drowsiness
hot flashes
blurred vision
difficulty sleeping
oxotremorine, arecoline, xanomelin = used for treatment of alzheimers disease; selective for mAChRs in brain
neurotransmitter dysfunction in alzheimers involves reduction in ACH, serotonin, NE, dopamine, and glutamate levels
SNN molecule
1,2,5-thiadiazole = non-classical bioisotere of ester
mimics ester
bioisotere
bioisoteres are functional groups or molecules that have chemical and physical similarities producing broadly similar biological properties
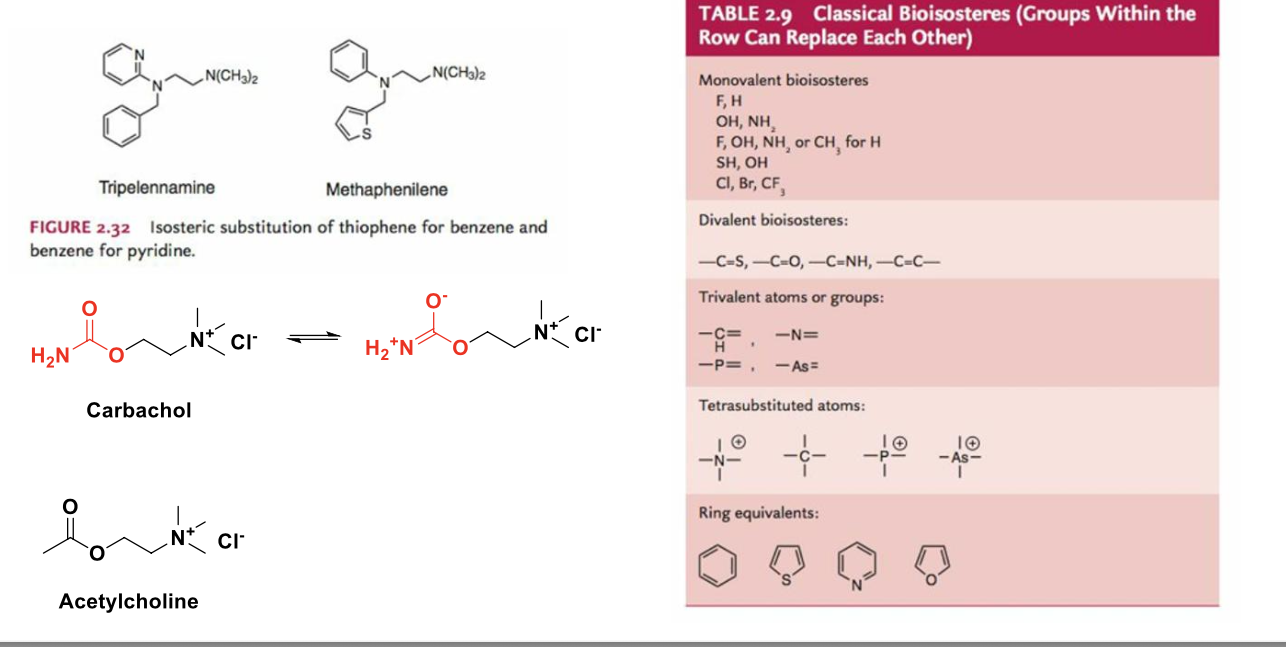
more bioisotere
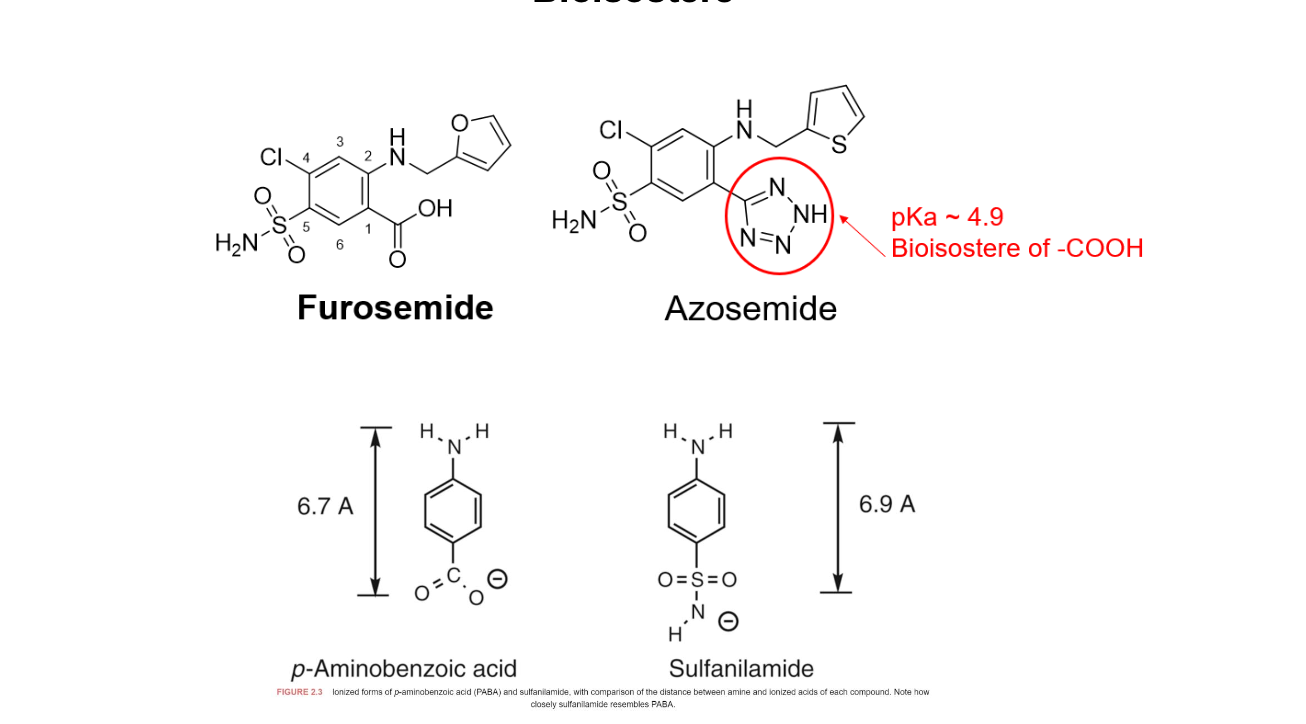
furosemide contains a COOH where azosemide replaces that acid with 1,2,4-triazol-3-one-like ring system that has a pKa around 4.9, similar to carboyxl group
PABA vs sulfanilamide
aniline nitroegn to COO- is nearly the same as they have similar pKa
structure-activity relationship of muscarinic agonists
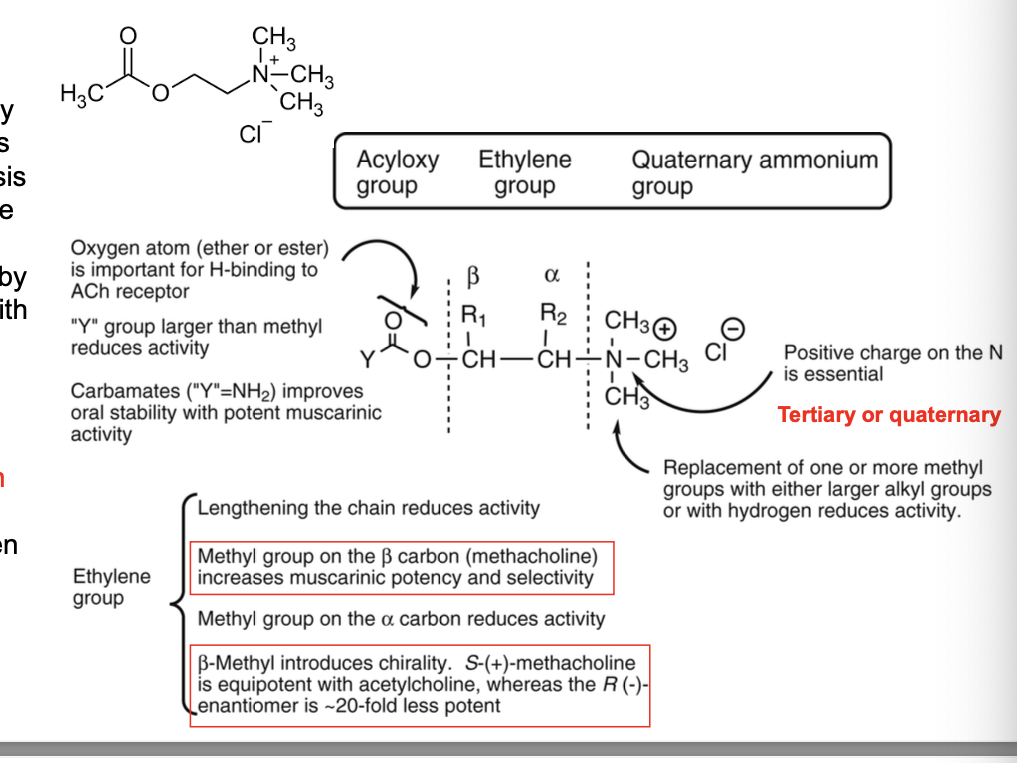
ACH is rapidly degraded by AChE and ubiquitous plasma esterases via the hydrolysis of the acetyl group; to improve the stability of ACH in the synpase, analogues have been developed by replacing the ester functionality with a hydrolytically more stable isotere
“rule of 5” by Ing state that there should be no more than 5 atoms btwn the cationic nitrogen and the terminal hydrogen atom (e.g. on the acetyl group of ACH) for optimal mAChR binding and stimulation
diagram
acyloxy group
oxygen atom (ether or ester) is important for H-binding to the ACH receptor
Y group larger than methyl reduces activity
carbamates (“Y”=NH2) improves oral stability with potent muscarinic activity
quaternary ammonium grp
positive charge on the N is essential (tertiary or quaternary)
replacement of one or more methyl groups with either larger alkyl group or with hydrogen reduces activity
ethlyene group
lengthening the chain reduces activity
methyl group on the β carbon (methacholine) increases muscarinic potency and selectivity
methyl group on the α carbon reduces activity
β-methyl introduces chirality. S-(+)-methacholine is equipotent with ACH whereas the R-(-)-enantiomer is ~20 fold less potent
nicotinic receptor agonists
nicotine = dibasic containing a weak basic pyridine nitrogen with pKA of 3.1 and pyrrolidine nitrogen with pKA of 8.0
nicotine dependence is primarily attributed to its binding to the α4β2 nicotinic receptor subtype which produces pleasurable feelings by releasing dopamine from central dopaminergic neurons
1st line treatments for smoking cessation include nicotine replacement therapy (NRT), varenicline oral tablets, and bupropion sustained-release (SR) tablets
nicotine replacement therapy (NRT) available in 5 different dosage forms
oral inhaler
chewing gum
oral lozenge
nasal spray
ER transdermal patch
oral formulation and nasal spray provide rapid delivery of nicotine while patch has slower onset of nicotine delivery but 24 hour duration
NRT delivers nicotine to CNS in a lower concentration and at much slower rate than tobacco smoke
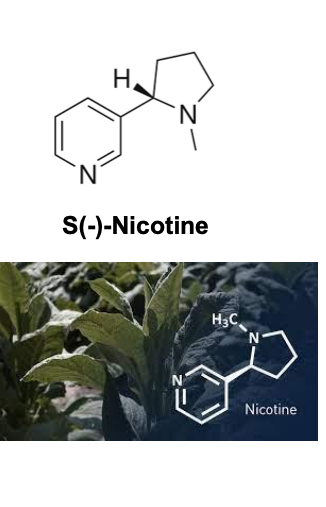
log(10) of levels of nicotine in plant sources
plants that contain nicotine
tomatoes
eggplant
potato
cauliflower
peppers
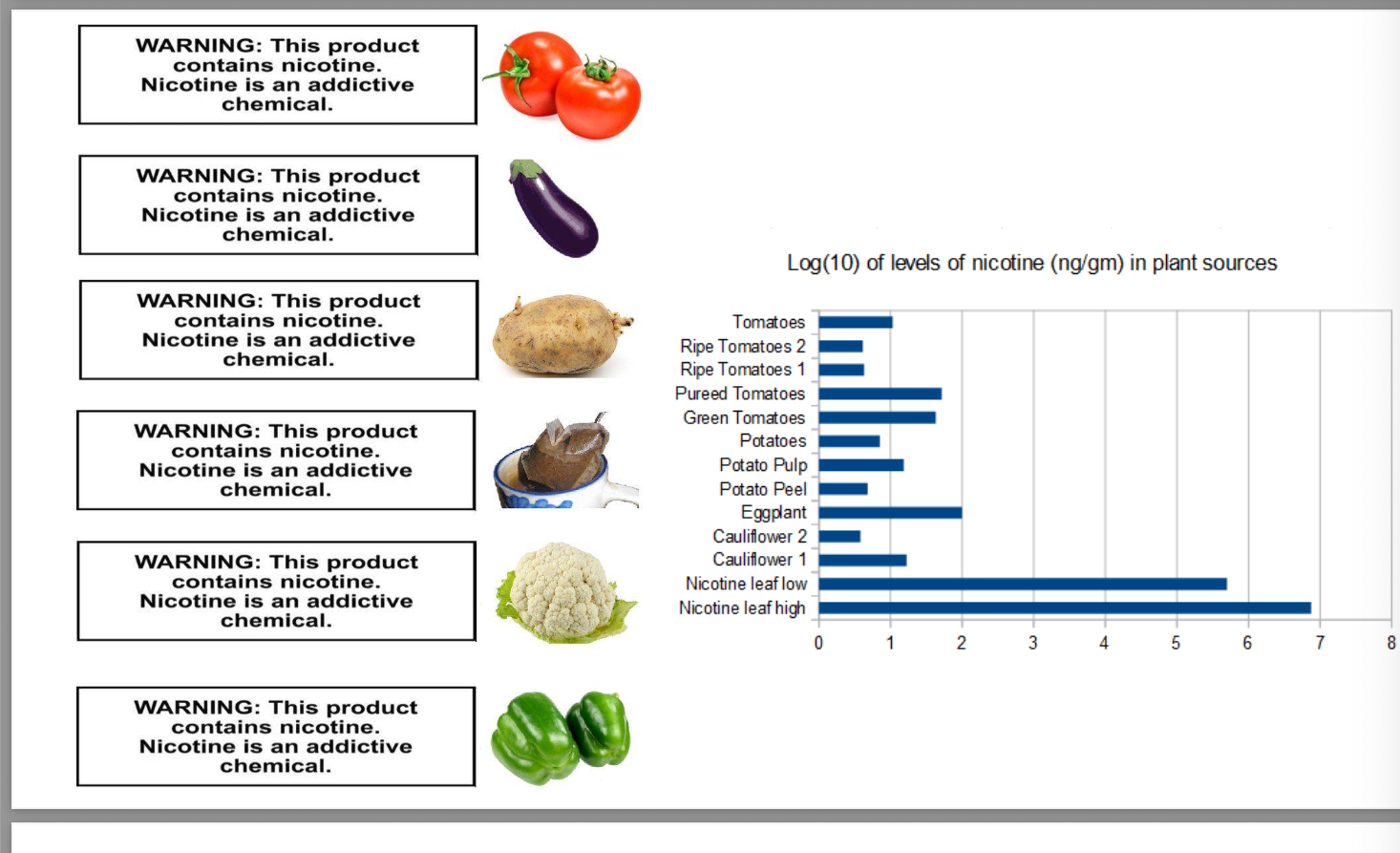
nicotinic receptor agonist/antagonist
bupropion = initially approved for the treatment of depression
12 hour sustained release tablet under brand name zyban
another first line-agent for smoking cessation
MOA = unclear but likely involves several mechanisms including re-uptake inhibition and NE re-uptake inhibition in the CNS and non-competitively blocks the activation of neuronal nAChRs by nicotine (nAChR antagonist)
varenicline = partial agonist at α4β2 and full agonist at α7 nAChRs
aid to smoking cessation treatment
developed from structural studies of the sub-structures of both morphine and cyticine
cyticine = naturally occuring α4β2 antagonist from laburnum species (golden rain tree)
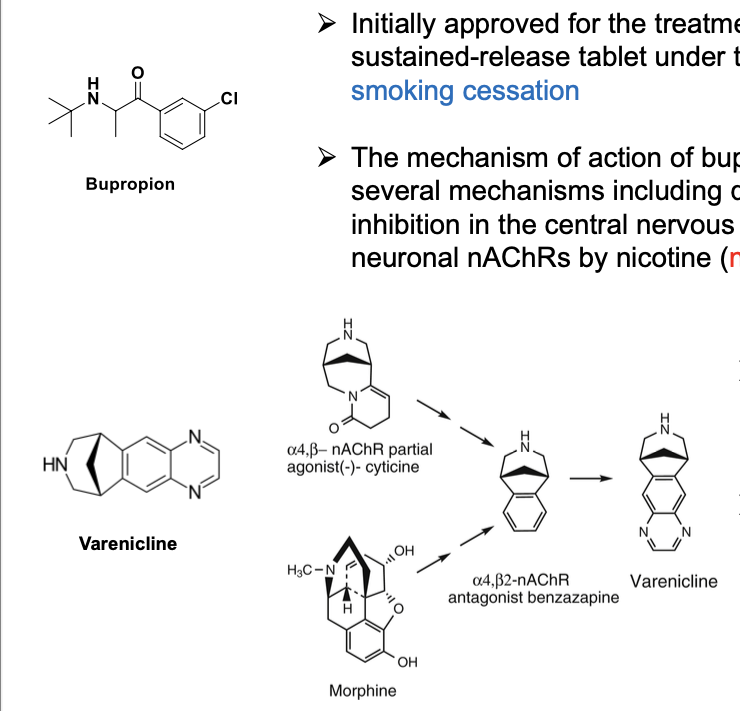
acetylcholine hydrolysis by AChE
acylation + deacylation
classification of acetylcholine esterase inhibitors
reversible inhibitors
carbamates
neostigmine
pyridostigmine
physostigmine
rivastigmine
non-carbamates
edrophonium
donepezil
galantamine
tacrine
irreversible inhibitors
organophosphates
sarin
malathion
diazinon

indirect cholinomimetics
acetylcholinesterase inhibitors (AChEIs), anticholinesterases, indirect cholinomimetics
inhibit acetylcholinesterase, but do NOT bind to cholinergic receptors
increase and prolonged acetylcholine-mediated muscarinic and nicotinic effects (equivalent ot muscarinic and nicotinic receptor agonists)
therapeutic applications
treatment of:
myasthenia gravis
glaucoma
alzheimers
insecticides
chemical warfare agents
reversible AChEIs are
those compounds (carbamates) that are substrates for and react with AChE to form an acylated enzyme which is MORE stable than the acetylated enzyme but still capable of undergoing hydrolytic regeneration (revesrible covalent) or
have nitro groups
those that bind to AChE with GREATER affinity than ACH but do NOT react with the enzyme as a substrate (non-covalent)
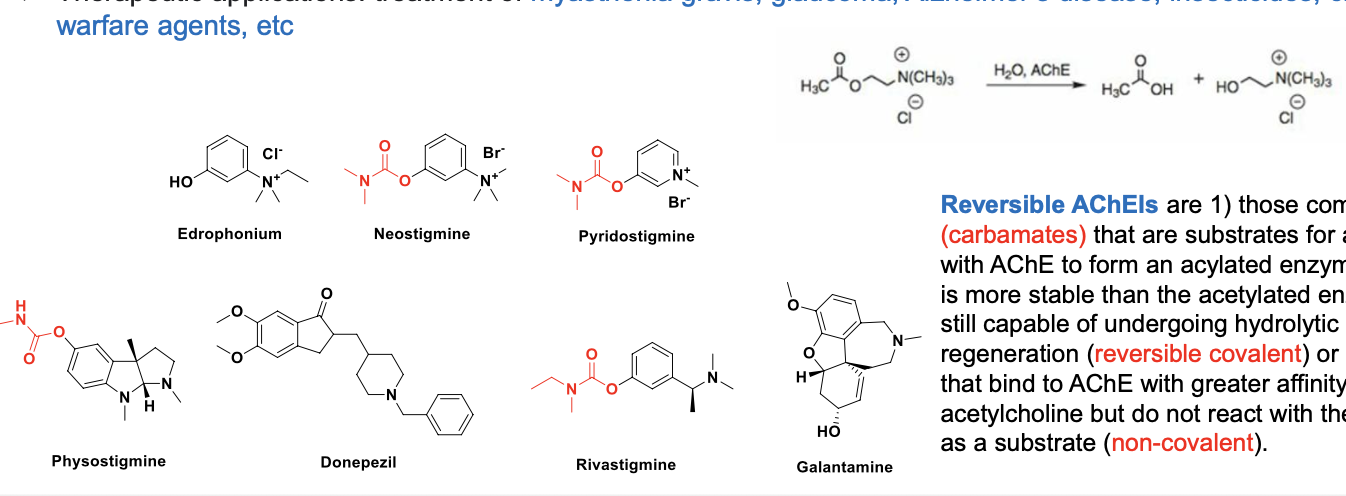
a prototype reversible carabamte AChE inhibitor: physostigmine
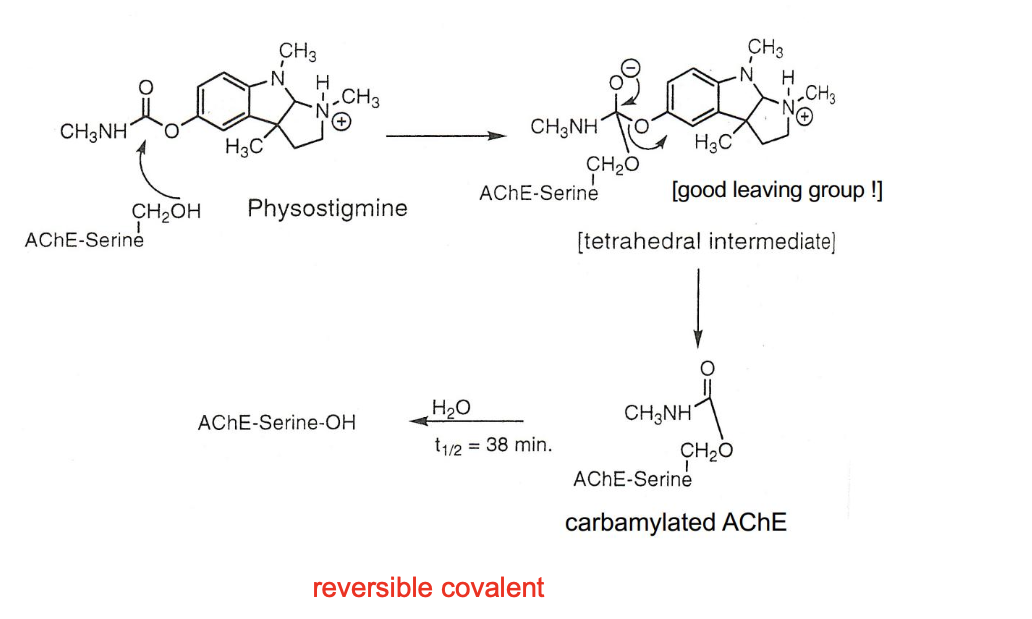
reversible covalent
steps
binding and nucleophilic attack
leaving group leaves (good LG - phenoxy/indolenium)
slow hydrolytic regeneration
physostigmine → tetrahedral intermediate → carbamylated AChE → back to AChE-serine-OH
indirect cholinomimetics
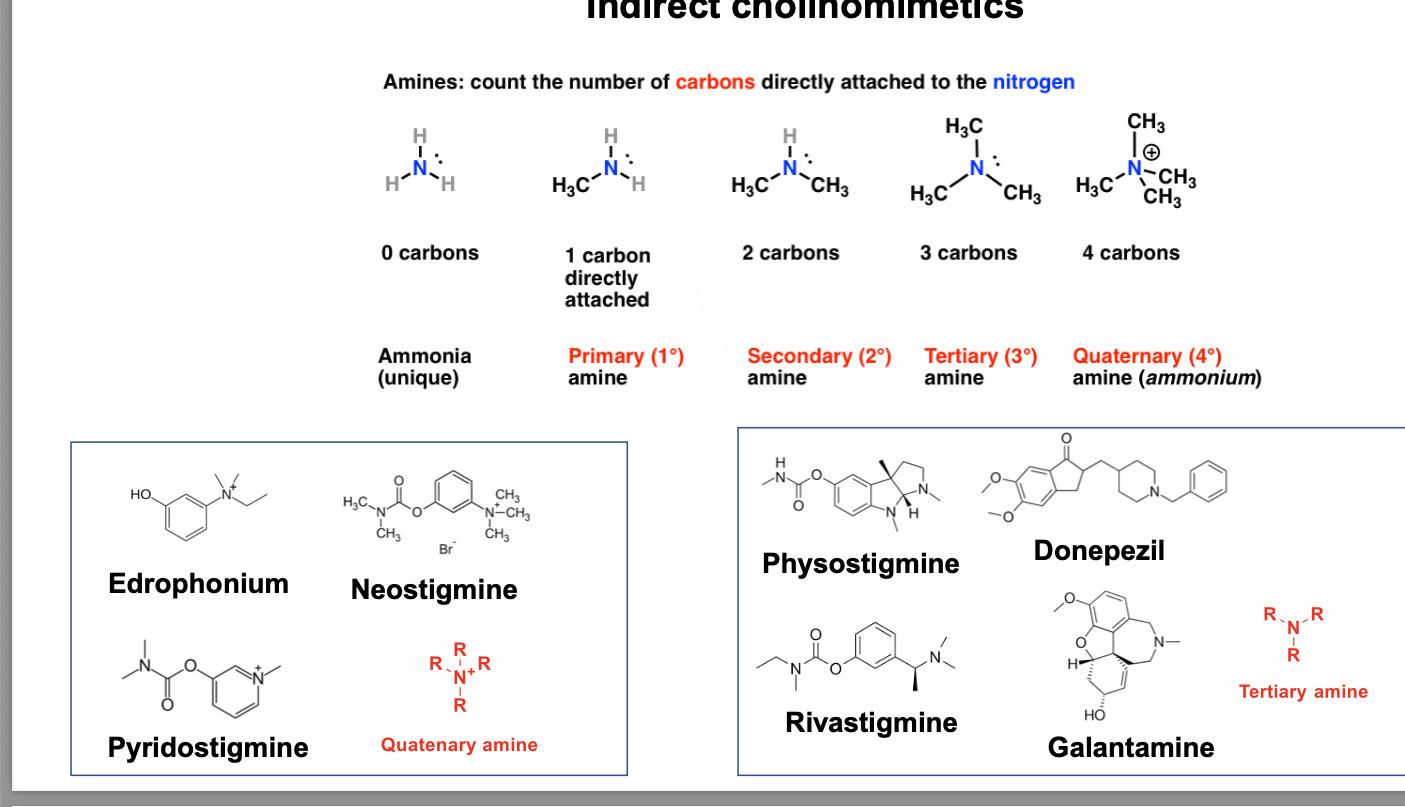
amines = count the number of carbons directly attached to the nitrogen
0 carbons = ammonia (unique)
1 carbon directly attached = primary amine
2 carbons = secondary amine
3 carbons = tertiary amine
4 carbons = quaternary amine (ammonium) pos. charge on N
tertiary amines
physostigmine
donepezil
rivastigmine
galantamine
quatenary amine
edrophonium
neostigmine
pyridostigmine
indirect cholinomimetics pt 2
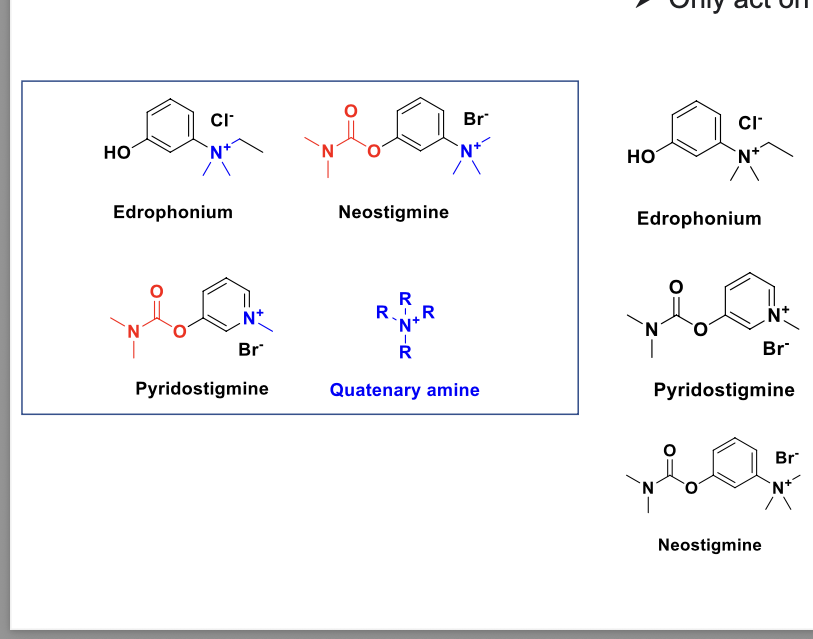
do NOT cross the BBB
only act on the PNS
edrophonium
used for the diagnosis of myasthenia gravis
shortest acting → onset 10-30 sec; duration 10 mins
pyridostigmine
used for treatment of myasthenia gravis
longest acting
neostigmine
used to reverse the anesthesia post op
treatment of myasthenia gravis
myasthenia gravis
autoimmune disease in which IgG1-dominant antibodies disrupt nicotinic acetylcholine receptors at NMJs (as well as muscle-specific kinase or lipoprotein-related protein) → results in muscle weakness that affects the eyes, face, head, and neck causing difficulities swallowing, speaking and eating; limb and respiratory muscles can also be affected
neostigmine and pyridostigmine prolong the action of ACH specifically at NMJ
indirect cholinomimetics (cholinesterase inhibitors)
cholinesterase inhibitors (ChEIs) work by preventing the breakdown of ACH which helps maintain activity at cholinergic synpases
while ChEIs have been shown to be effective in large-scale studies; effect sizes are condiered modest → considered to by “symptomatic” treatments
alzheimers is characterized by low levels of ACH, serotonin, NE, dopamine and glutamate
donepezil, rivastigmine, galantamine = indirect cholinomimetics which are used to treat alzheimers; improves symptoms (do NOT prolong survival)
oxotremorine, arecoline, and xanomeline = muscarinic agonists
tacrine was approved for AD but use is limited due to hepatotoxicity
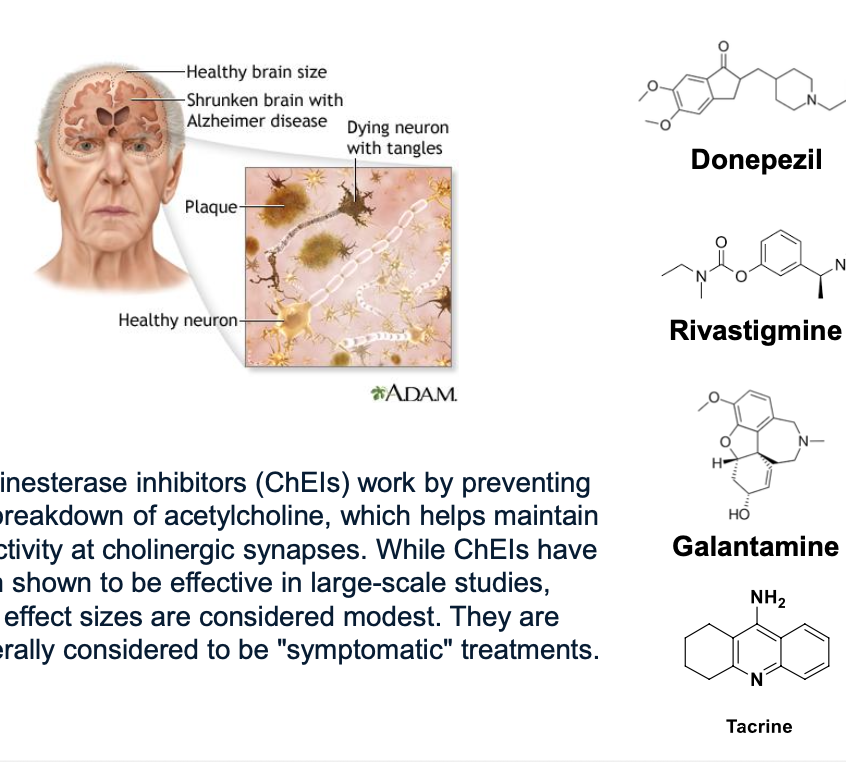
indirect cholinomimetics (cholinesterase inhibitors) pt 2
physostigmine = reversible cholinesterase inhibitor used to treat glaucoma and anticholinergic toxicity (antidote of atropine)
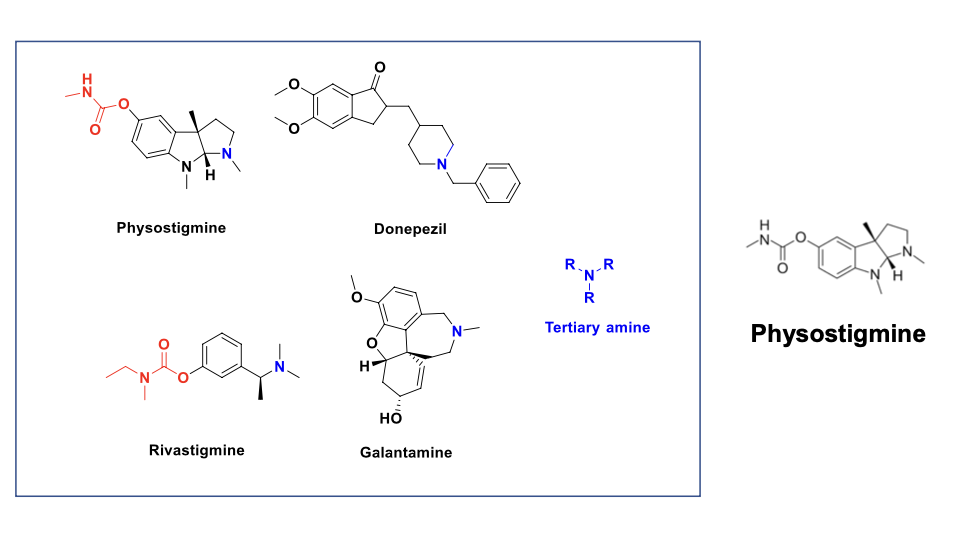
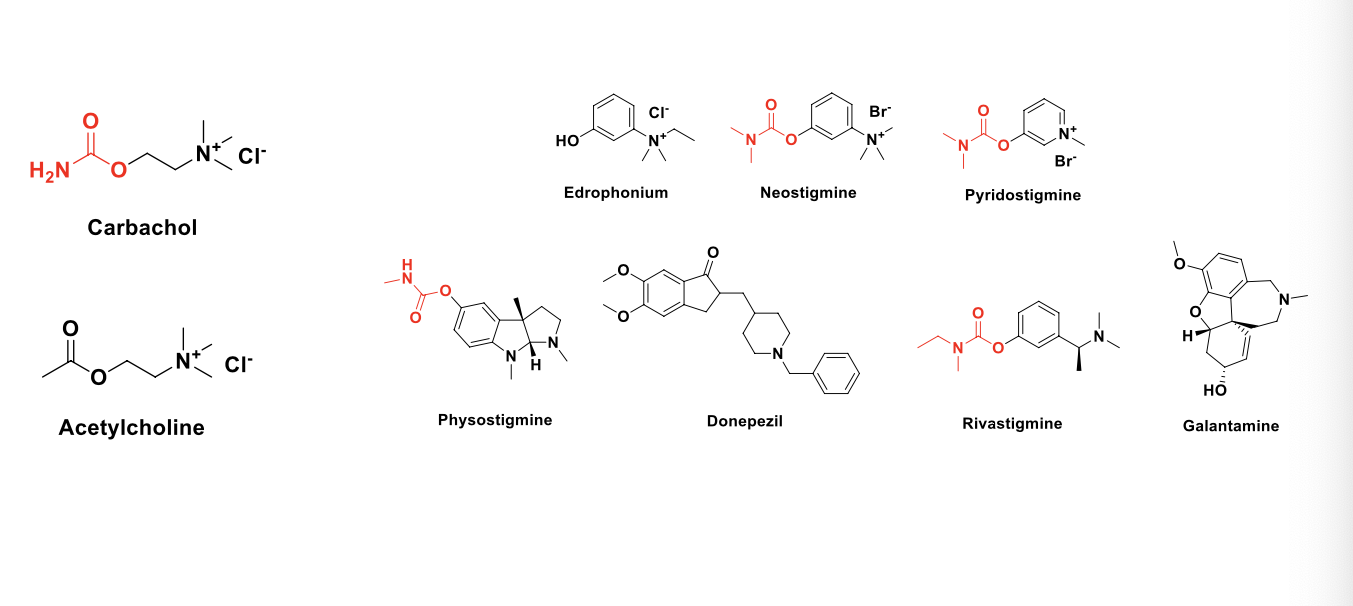
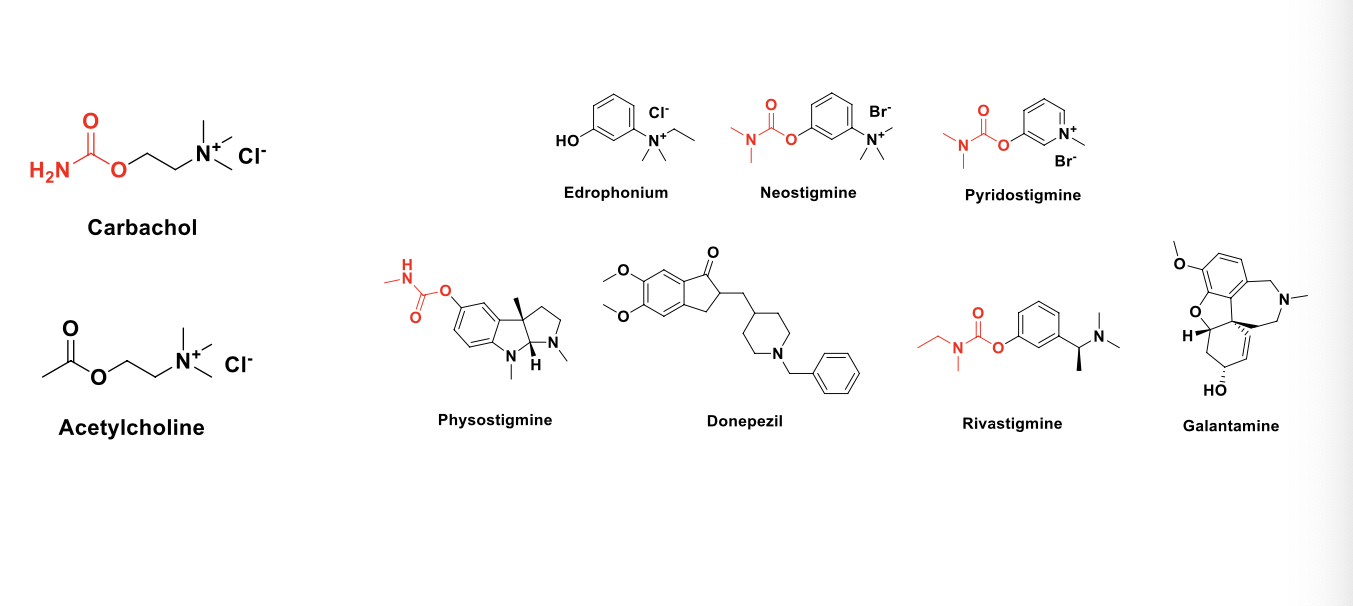
what are the MOA of the following drugs?
direct cholinomimetics (receptor agonists)
ACH
carbachol
indirect cholinomimetics (acetylcholinesterase inhibitors)
edrophonium
neostigmine
pyridostigmine
physostigmine
donepezil
rivastigmine
galantamine
irreversible AChEIs as chemical weapons
phosphonate esters are chemically more stable towards hydrolysis (harder to hydrolyze) and metabolically more resistant to esteraess and phosphatases (phosphate esters are even more stable than many amides)
rate of hydrolysis of the phosphorylated enzyme = more slower than that of the carbamylated enzyme and its rate is measured in hours. b/c the duration of action of these compounds if much longer than carbamate esters → referred to as irreversible inhibitors of AChE
aging is the result of hydrolysis of 1 or more of the phosphoester bonds while leaving the AChE phosphorylated
this rxn affords an anionic phosphate in which the phosphorus atom is much less electrophilic → less likely to undergo hydrolytic regeneration than original phosphoester
simple terms
after an organophosphate inhibits AChE; one of its side groups can hydrolyze off → this change leaves a negatively charged phosphate on the enzyme, making the phosphorus less “electron loving” (electrophilic) → b/c its less reactive, antidotes can NO longer attack as easily so enzyme CANNOT be regen
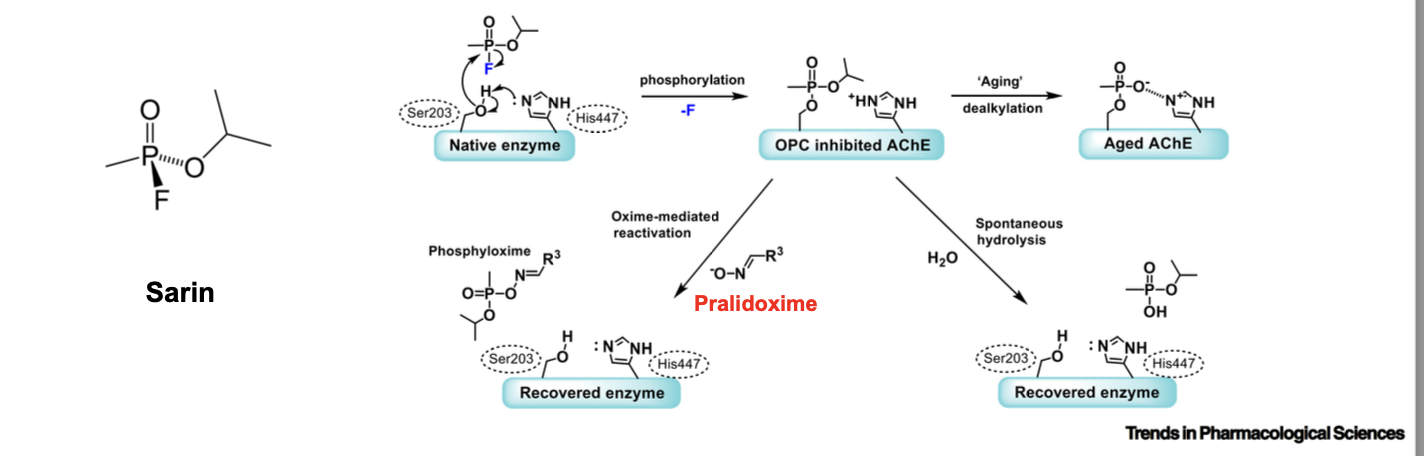
novichok nerve toxins
V class of nerve toxins were less volatile and to persist in the area that they were dispersed be used in warfare
VX is more potent than sarin
exposure of 10 mg or more can be fatal
was used to kill Kim-Jong-nam (brother of kim jog-un) in malaysia in dec of 2017
novichok class of nerve toxins was developed in russia
median lethal dose for A-234 is 0.2 mg (5000 lethal doses in a gram) and is below 0.1 mg for A-230 (10,000 lethal doses in a gram)
novichok agents = organophosphorus compounds, similar to sarin and VX, which inhibit the enzyme acetylcholinesterase and cause a biochemical logjam that crippled the nervous system
symptoms range from sweating and twitching to seizures and inability to breathe
irrversible modification of acetylcholinesterase by nerve gas agents such as A-230 CANNOT be reversed by treatment with 2-PAM to allow for regeneration of active enzyme b/c of increase stability of phosphonate esters
VX/VR = V series nerve agents
the As = novichok agents
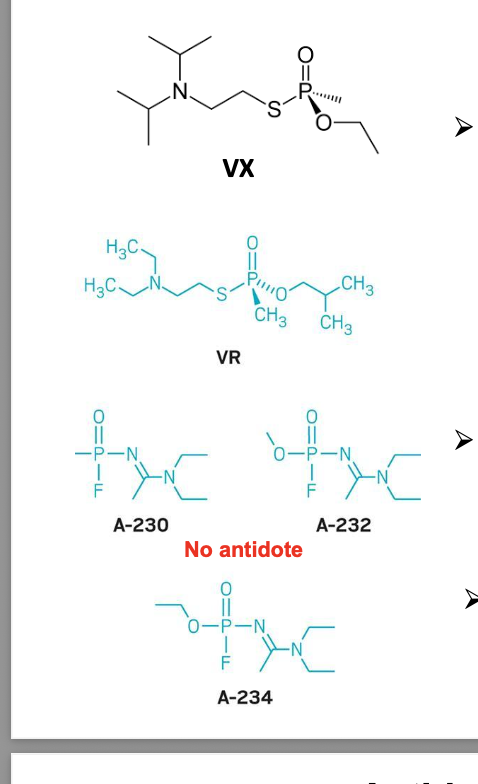
antidote for organophosphate poisoning
pralidoxime (protopam or 2-PAM) = strong nucleophile that competes with AChE for the phosphorus group of the inhibitor (suicide inhibition of the inhibitor) and enzyme is restored
protopam = antidote to treat organophosphate poisoning; however, must be given within 24-36 hrs of poisoning otherwise enzyme becomes resistant to the antidotal effects of protopam b/c of “aging”
aging = hydrolysis of 1 or more of the phosphoester bonds while leaving the AChE phosphorylated
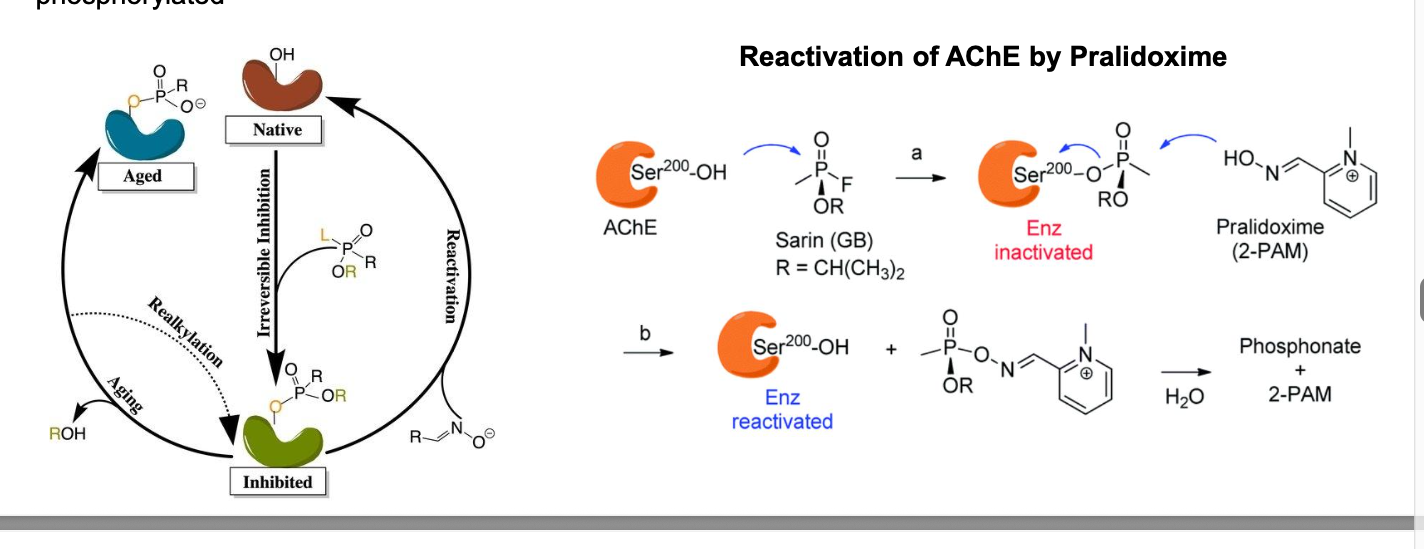
irreversible inhibitors of acetylcholinesterase used as insecticides
malathion
parathion
schradan
dichlofenthion
chlorpyrifos
carbaryl
carbamate insecticides = slowly reversible inhibitors of AChE
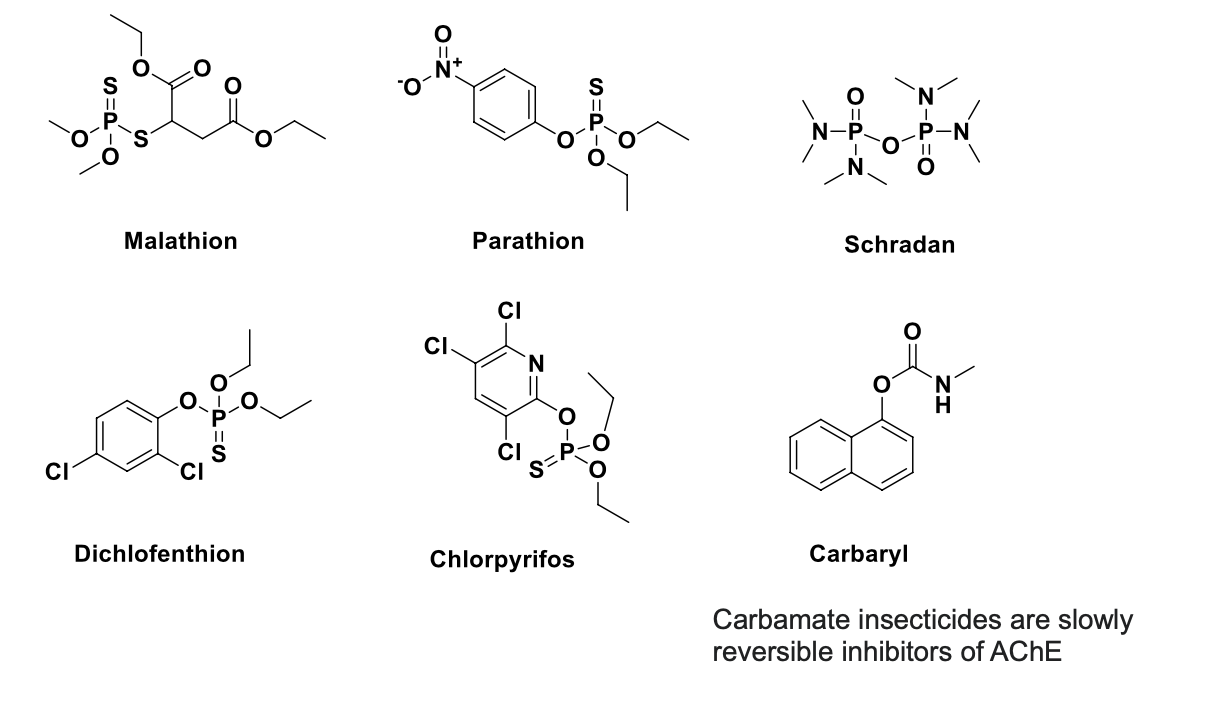
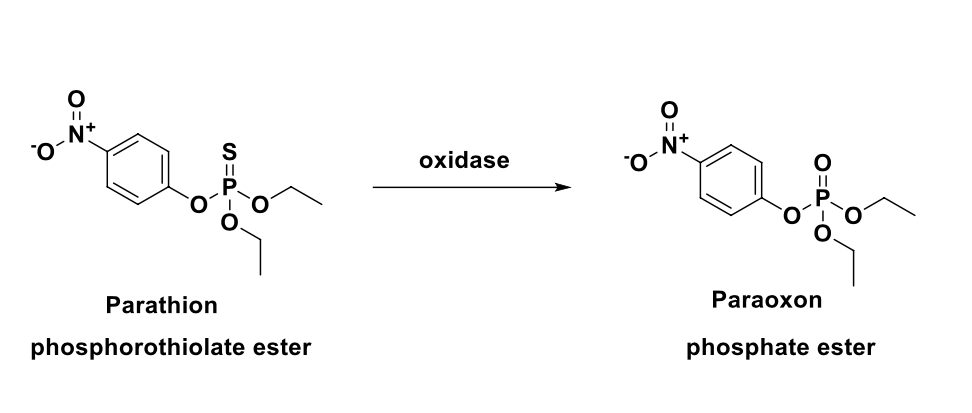
MOA of parathion
parathion acts on the enzyme acetylcholinesterase indirectly
after insect (or human) ingests parathion, oxidase replaces the double bonded sulfur with oxygen to give paraoxon
as a pesticide, parathion is generally applied by spraying; often applied to cotton, rice and fruit trees
the usual concentration of ready-to-use solutions are 0.05%-0.1%; chemical is banned for many food crops
case study
a 23 year old woman is brought to the ER after deliberately ingesting a bottle of organophosphate insecticide
a) why is it important to ID the specific product this woman ingested
b) what is the appropriate treatment for this ingestion
a) 2 types of insecticides in this class: carbamate insecticides and organophosphate insecticides. the carbamate insecticide and organophosphate insecticides. the carbamate insecticides are “reversible” and inhibit AChE in a fashion identical to other carbamoylating agents (physostigmine and neostigmine) while the organophosphate insecticides inhibit AChE in an irreversible manner by alkylphosphorylation. the organophosphate inhibition of AChE is initially reversible but “ages” into an enzyme inhibition that is resistant to hydrolysis and reactivation. the symptoms of poisoning from both insecticides resemble each other but poisoning from an OP insecticide will benefit from early admin of AChE reactivator
b) atropine in sufficient dosages (large doses may be required) effectively antagonizes the effects at muscarinic receptor sites. atropine is virtually WITHOUT effect against the peripheral neuomuscular junction (nicotinic) effects. the nicotinic effects of acute OP poisoning can be reverse by pralidoxime (2-PAM), a cholinesterase re-activator. the reactivation of AChE is most pronounced at skeletal NMJ b/c pralidoxime has weak anti-cholinesterase activity, it is NOT recommended for the treatment of overdosage with physostigmine or neostigmine (reversible inhibitors) or poisoning with the carbamoylating insecticides such as carbaryl
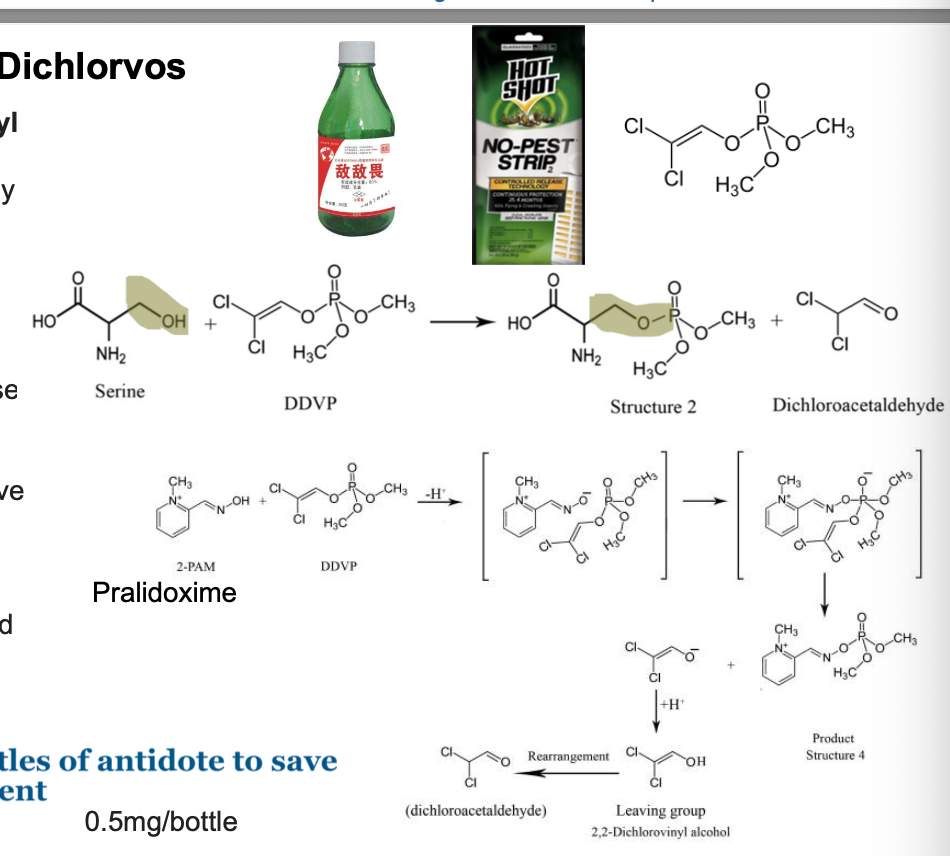
dichlorvos
dichlorvos (2,2-dichlorovinyl dimethyl phosphate, DDVP) is an OP widely used as an insecticide to control household pests and protecting stored products from insects
dichlorvos inhibit acetylcholinesterase associated with/the nervous system of insects; claimed to damage DNA of insects
antidotes for dichlorvos are atropine and pralidoxime
acetylcholine antagonists - muscarinic antagonists
muscarinic antagonists
anticholinergics
antimuscarinics
cholinergic blockers
antispasmodics
parasympatholytics
act as competitive (reversible) antagonists and produce opposite pharmacological effects of muscarinic agonists
responses of muscarinic antagonists include:
decreased constraction of smooth muscle of the GI and urinary tracts
dilation of pupils
reduces gastric, mucociliary, and salivary secretions (treat overactive bladder)
muscarinic antagonists are common components of cold and flu remedies that act to reduce nasal and upper respiratory tract secretions
acetylcholine antagonists - muscarinic antagonists pt 2
atropine = used for treatment of bradycardia and as pre-operative agent to reduce secretions before surgery
antidote for anticholinesterases (OP nerve agents and insecticids) and muscarine
scopolamine = most used for treatment of motion sickness
available in transdermal patch (behind the ear) and oral pills to prevent nausea and vomitting
acetylcholine antagonists - muscarinic antagonists pt 3
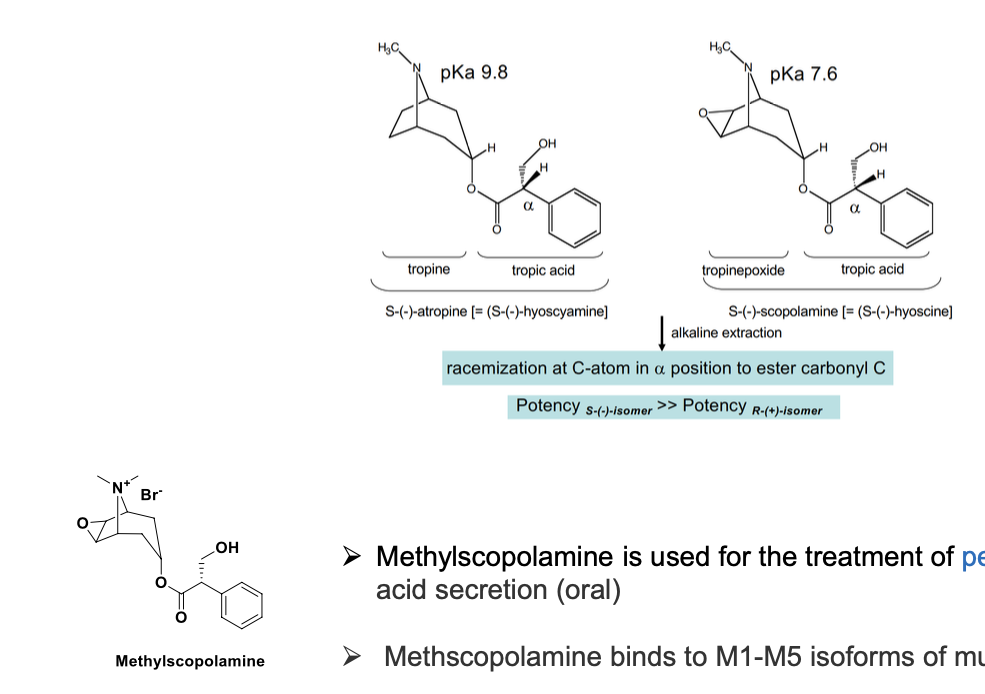
methylscopolamine is used for the treatment of peptic ulcer by reducing stomach acid secretion (oral)
methscopolamine binds to M1-M5 isoforms of muscarinic receptors
S isomer of methylscopolamine = more potent than R isomer
S has higher pKa and R has lower pKA
sea sickness - dramamine
diphenhydramine (benadryl)
1st generation histamine H1-receptor antagonists (blockers)
used to prevent and treat nausea, vomiting, and dizziness caused by motion sickness
also used to relieve symptoms of allergy, hay fever, and common cold
8-chlorotheophyline
also known as 1,3-dimethyl-8-chloroxanthine is a stimulant drug of the xanthine chemical class w/physiological effects similar to caffeine
diphenhydramine reduces nausea but causes drowsiness, and the stimulant properties of 8-chlorotheophylline help reduce that side effect
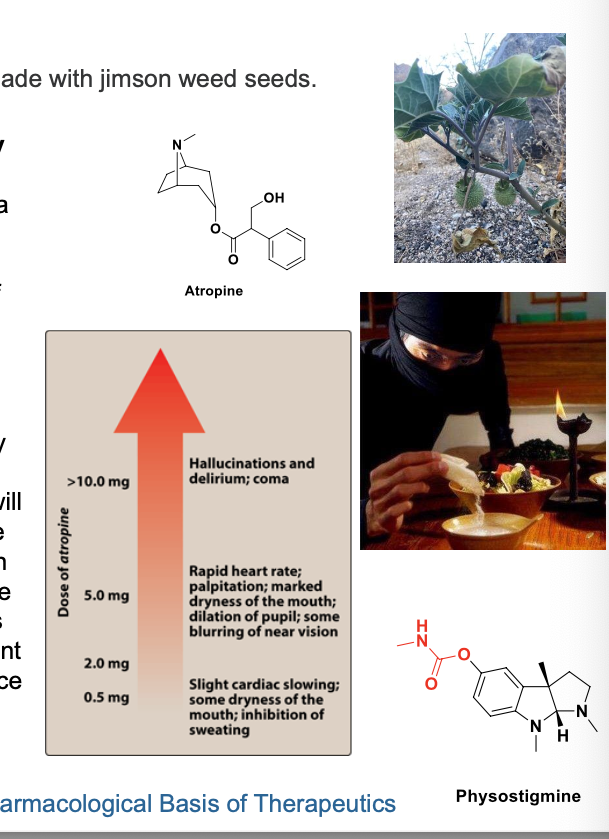
case study
a 14 year old boy is brought to the ER after drinking a tea made with jimson weed seeds
a) what is jimson weed and what are the likely symptoms this boy may experience?
b) if his symptoms are serious and life threatening, what is the appropriate treatment?
a) jimson weed = common plant that contains atropine and other belladonna alkaloids. seeds contain high concentrations of these alkaloids and poisoning results from their ingestion. symptoms of various doses of atropine are and are predictable as consequence of muscarinic receptor antagonism.
b) measures to limit absorption of the atropine should be initiated WITHOUT delay if the poison had been taken orally. for symptomatic treatment, slow IV injection of physostigmine (an AChE inhibitor) will increase ACh at muscarinic receptors. physostigmine will rapidly abolish the delirium and coma caused by high doses of atropine but carries some risk in mild atropine intoxication. b/c physostigmine is metabolized rapidly, the pt may again lapse into a coma within 1-2 hours and repeated doses may be needed. if marked excitmenet is present and more specific treatment is NOT available, a benzodiazepine = more appropriate treatment to induce sedation and prevent seizures. support of respiration and control of hyperthermia may be necessary.
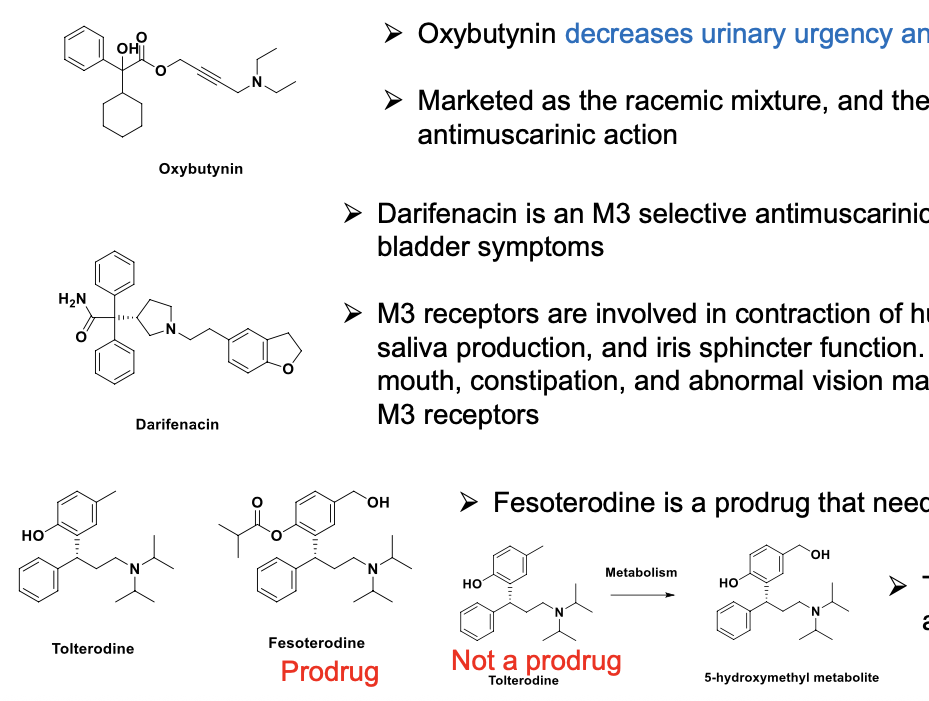
muscarinic antagonists (overactive bladder)
oxybutynin decreases urinary urgency and frequency
marketed as the racemic mixture and the R-isomer is responsible for the anti-muscarinic action
darifenacin is an M3 selective anti-muscarinic that acts quickly to improve overactive bladder symptoms
M3 receptors are involved in contraction of human bladder and GI smooth muscle, saliva production and iris sphincter function. adverse drug effects such as dry mouth, constipation and abnormal vision may be mediated thru antagonism of M3 receptors
fesoterodine = pro-drug that needs to be hydrolyzed to active form
5-hydroxymethyl metabolite is active as tolterodine
acetylcholine antagonists - muscarinic antagonists (overactive bladder)
aminoalkyl ester
a highly selective M3 antagonist used for the treatment of overactive bladder w/less side effects
trospium is the only quaternary amine anti-muscarinic available for overactive bladder
ER doage form has elimination half-life of 35 hours
cevimeline = muscarinic agonist

drugs for the treatment of overactive bladder
antimuscarinics (anticholinergics)
oxybutynin
tolterodine
solifenancin
darifenacin
fesoterodine
trospium
β3 adrenergic agonists
mirabegron
vibegron
combo therapy
mirabegron/solifenacin
botulinum toxin
injection directly to bladder muscle
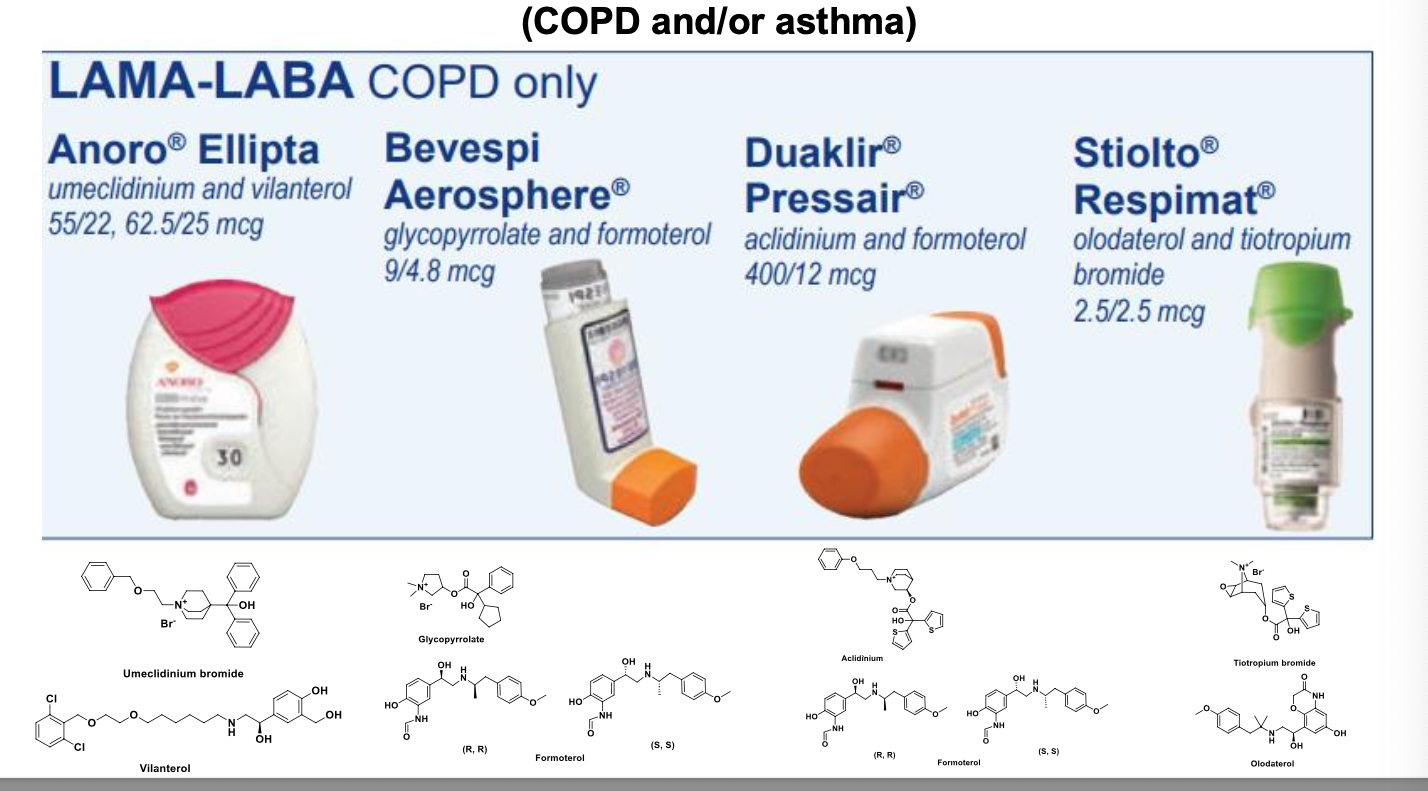
drugs for the treatment of COPD/asthma
COPD/asthma
β2 adrenergic agonists (bronchodilators)
short-acting β agonists
long-acting β agonists
anticholinergics (muscarinic antagonists)
corticosteroids
combo inhalers
acetylcholine antagonists - short acting muscarinic antagonists (COPD and/or asthma)
ipratropium is poorly absorbed from the lungs and the quintenary ammonium prevents it from crossing the BBB and producing undesired CNS side effects (inhaler or nebulizer)
15 mins onset of action and a short duration of action <4 hr
used as an inhalant to product dilation of bronchial smooth muscle in COPD
also used as nasal spray to treat rhinorrhea (persistant running nose) that is associated with common cold
SAMA and SABA-SAMA
atropine showed as comparison (tertiary vs quartenary)

acetylcholine antagonists - long-acting muscarinic antagonists (COPD and/or asthma)
aclidinium bromide was approved for the inhalation treatment of COPD
long acting bronchodilator (>24 hr) with a quick onset of action (inhalation)
quaternary amine
umeclidinium = dry powder inhalant and used with vilanterol (long-acting β2 adrenergic agonist) in 2 different formulation for the treatment of COPD (inhalation)
selective for M3 muscarinic receptor
quaternary amine
glycopyrrolate = aminoalcohol ester anticholinergic
used for treatment of COPD (inhalation)
tiotropium = relief of bronchospasms associated with COPD
30 mins onset and a long duration of action (>24 hr) (inhalation)
mainly acts on M3 muscarinic receptors
administered by oral inhalation of an aerosol solution (spiriva respimat) or dry powder inhaler (spiriva handihaler); do NOT take orally
acetylcholine antagonists - long-acting muscarinic antagonists (COPD and/or asthma) pt 2
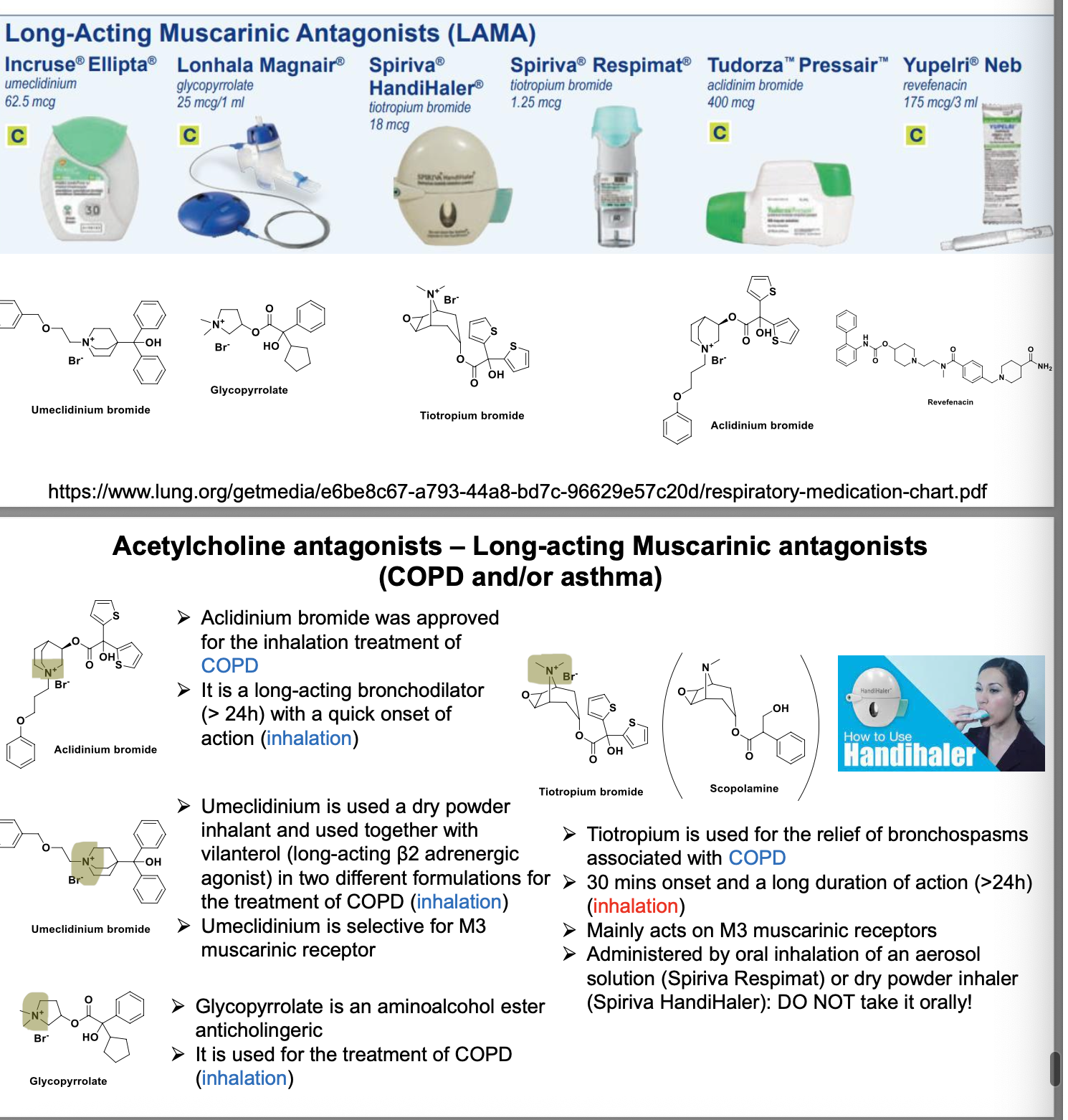
revefanacin = long acting muscarinic antagonist (>24 hr) for treatment of COPD (inhalation)
similar affinity to the subtyles of muscarinic receptors M1 to M5
in the airways, exhibits pharmacological effects thru inhibition of M3 receptor at the smooth muscle leading to bronchodilation
acetylcholine antagonists - muscarinic antagonists
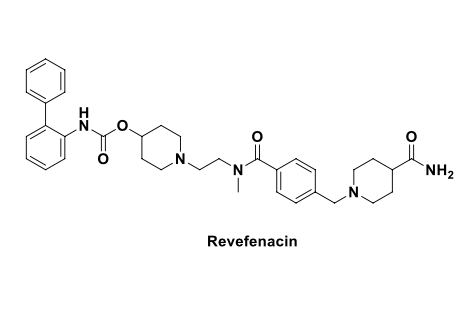
combo of chlordiazepoxide and clidinium used along with other meds to treat peptic ulcers, irritable bowel syndrome (IBS = condition that causes stomach pain, bloating, constipation and diarrhea) and entercolitis (swelling in the intestines); approved in 2021 (oral) (methylscopolamine is also used for the treatment of peptic ulcer as oral drug)
works by reducing muscle spasms in the GI tract and decreasing production of stomach acid
benzatropine = oral drug to treat symptoms of parkinsons
selective M1 msucarinic acetylcholine receptor antagonist
acetylcholine antagonists - long-acting muscarinic antagonists (COPD and/or asthma)
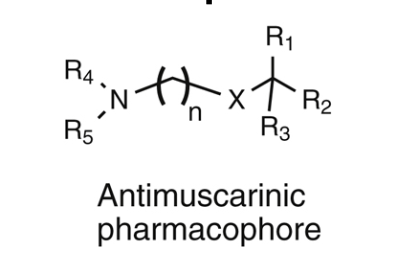
structure-activity relationship of muscarinic antagonistss
R1/R2 should be carbocyclic or heterocyclic rings and bind to the mAChR thru hydrophobic and/or π stacking interactions with active site Tyr, Phe, and Trp residues that lie outside of the ACH binding pocket. optimal potency is observed when one ring is aromatic and the other is saturated. typically 5 or 6 membered ring substituents are optimal. analogues containing larged ring structures, such as naphthalene, tend to be inactive, perhaps due to increased steric bulk
the R3 substituent = variable with hydrogen, hydroxy, hydroxymethyl, or carboxamide all acceptable. H-bonding hydroxy or hydroxymethyl subsittuents are optimal and can be a subsitutent on the R1 or R2 ring systems. these groups interact with a conserved Asn residue within the mAChR active site thru 2 hydrogen bonding inreactions.
the connecting functional group “X” in most analogues is an ester, mimicking ACH. however ether and carbon X moietites also produce active compounds. the nature of the connecting groups allows antimsucarinic agents to be chemically classified as aminoalkyl esters, aminoalkyl ethers, or aminoalcohols. nitrogen is typically a quaternary ammonium speicies (most potent) or tertiary amine. tertiary amine will be extensively protonated at physiological pH to generate the cationic species needed for ionic interactions w/the conserved active site Asp residue.
R4/R5 are typically small alkyl groups such as methyl, ethyl, propyl, or isopropyl. some compounds contain a cyclic motif to yield the quaternary nitrogen
due to molecular flexibility, the distance between the nitrogen aotm of the tertiary of quarternary amine and the ring-substituted carbon seems to be less critical for activity. normally, the linking chain is 2-4 carbons (optimal)
nicotinic acetylcholine antagonists
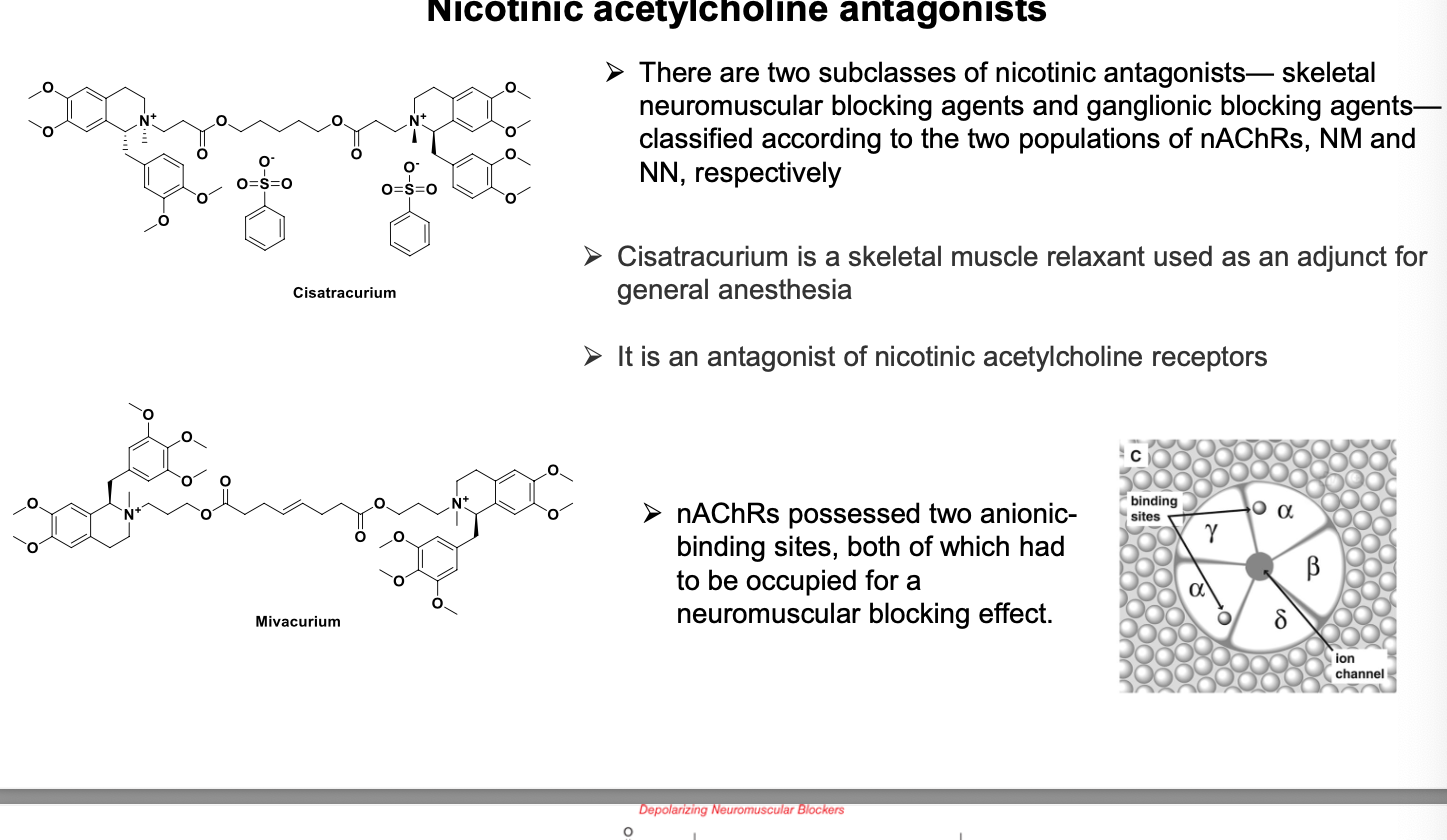
2 subclasses of nicotinic antagonists - skeletal neuromuscular blocking agents and ganglionic blocking agents - classified according to the 2 populations of AChRs, NM and NN, respectively.
cisatracurium = skeletal muscle relaxant used as an adjunct for general anesthesia
an antagonist of nicotinic acetylcholine receptors
nAChRs posses 2 anionic binding sites, both of which have to be occupied for neuromuscular blocking effect
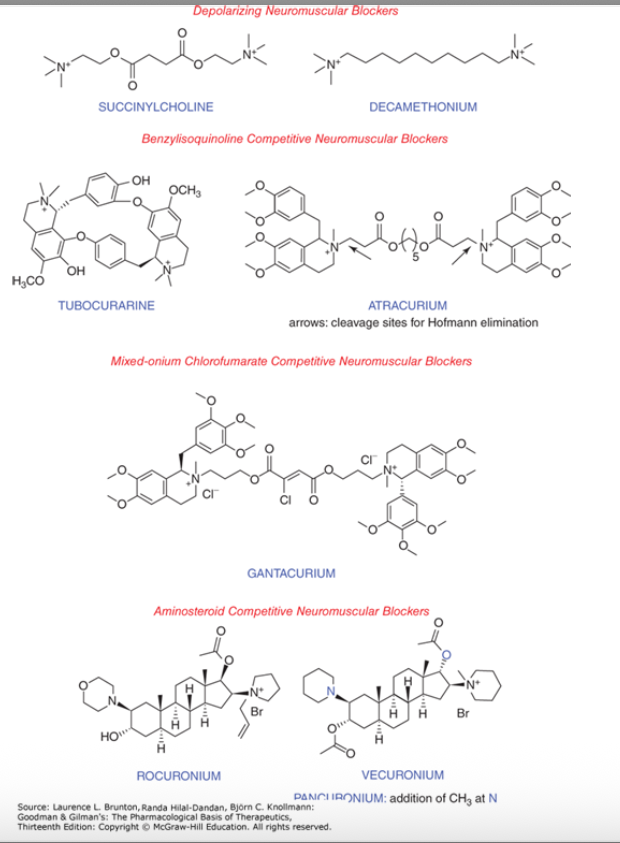
neuromuscular blocking agents
depolarizing neuromuscular blockrs
succinylcholine
decamethonium
benzylisoquinoline competitive neuromuscular blockers
tubocurarine
atracurium
mixed-onium chlorofumarate competitive neuromuscular blockers
gantacurium
aminosteroid competitive neuromuscular blockers
rocuronium
vecuronium