1.1 Inside the atom
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
What 2 components does every atom contain?
a positively charged nucleus composed of protons and neutrons
electrons that surround the nucleus
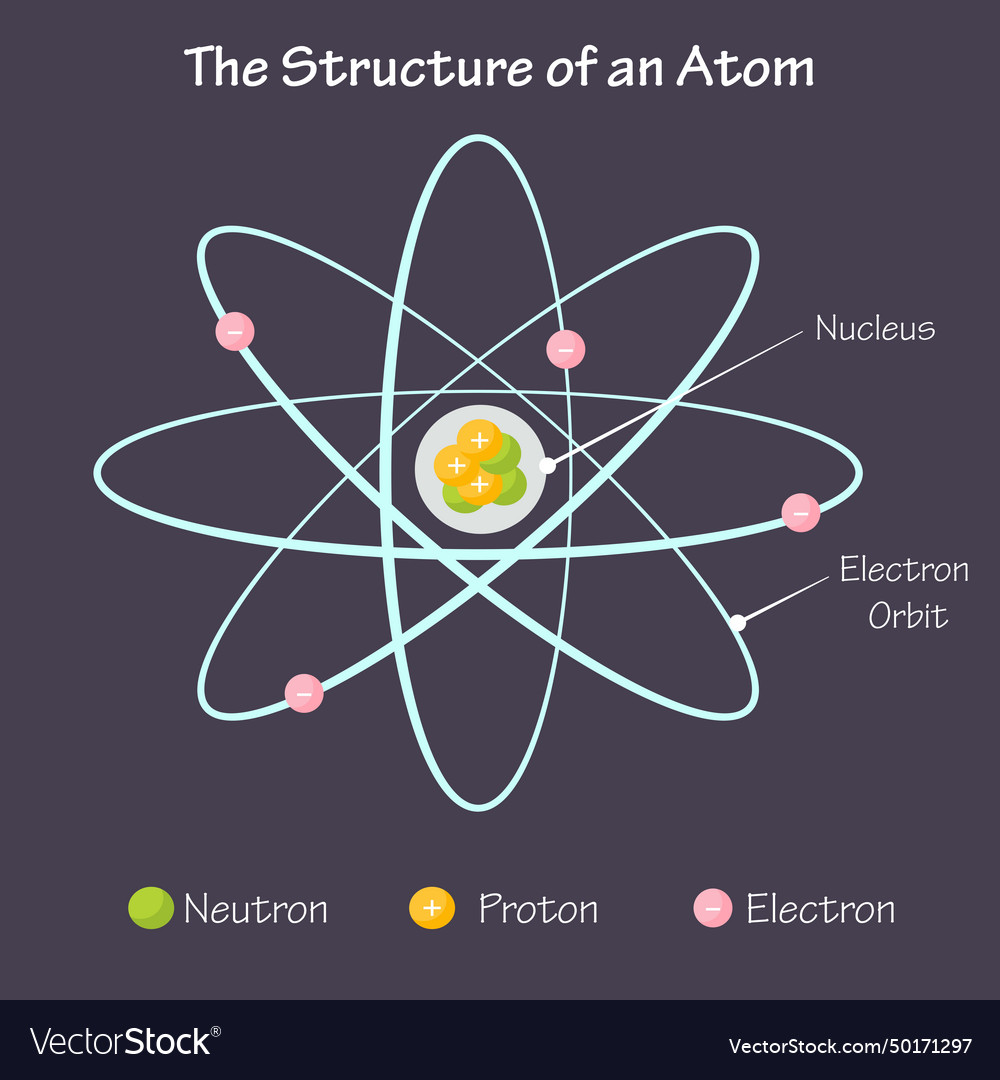
nucleon
proton or neutron in the nucleus
What force of attraction holds electrons and nucleus together?
electrostatic
The electron has a much smaller mass than the _____ or the _______
proton
neutron
The proton and the neutron have almost __ mass.
equal
The electron has equal and __ charge to the proton.
opposite
The neutron is __.
uncharged
Isotope
atoms with the same number of protons and different number of neutrons
nuclide
a specific isotope of an element characterized by the number of protons and neutrons in its nucleus
specific charge
charge divided by mass
particle with highest specific charge
electron