Module 5: Electrons and Light
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
electron configuration
the arrangement of electrons in the energy levels, sublevels and orbitals of atoms
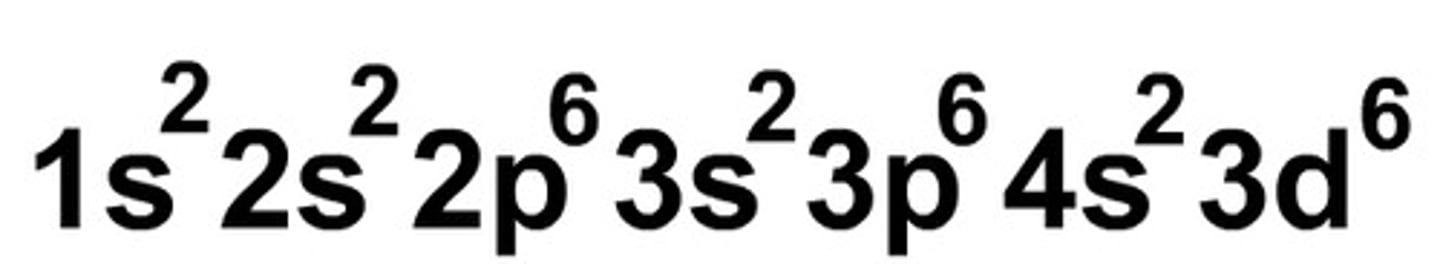
Aufbau Principle
states that each electron occupies the lowest energy orbital available

Pauli Exclusion Principle
maximum of two electrons may occupy an atomic orbital, but only if they have opposite spins

Hund's Rule
every orbital in a sublevel must have an electron before any orbital gets two, and all electrons in singly occupied orbitals have the same spin.
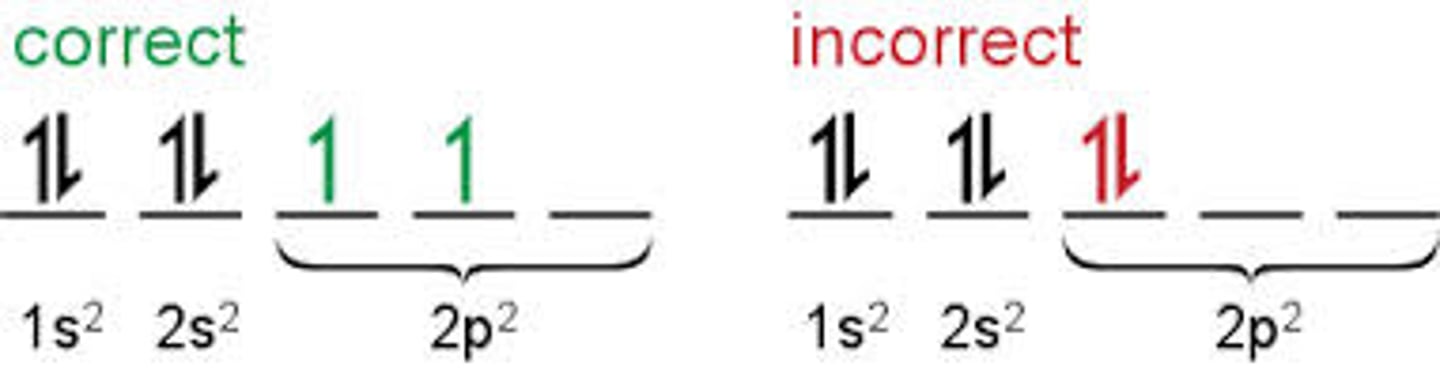
electron cloud
a region around the nucleus of an atom where all electrons are likely to be found (most probable location)

orbitals
regions within electron cloud where electrons orbit the nucleus; can hold a maximum of 2 electrons
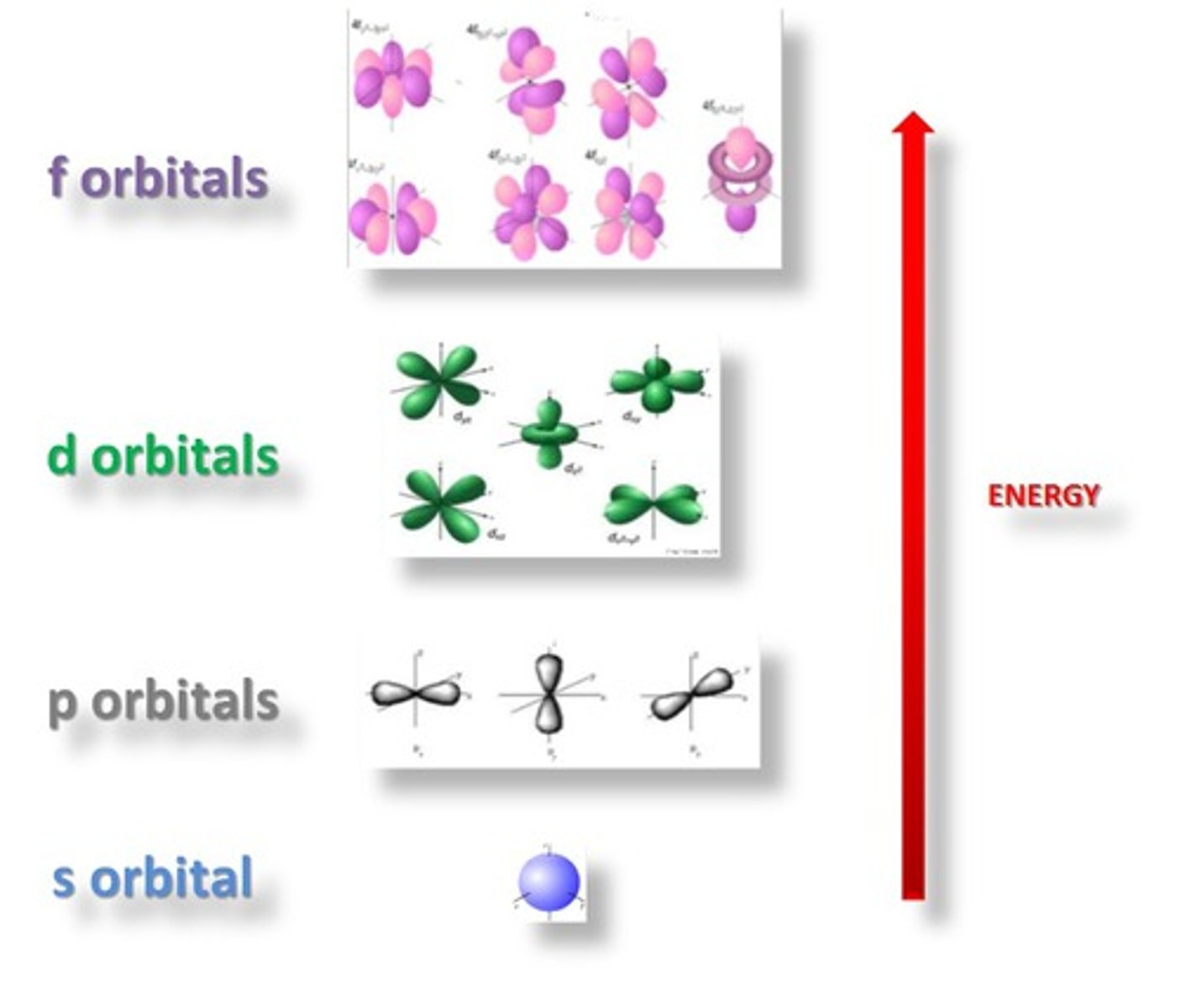
s sublevel
spherical orbitals; first to fill for any energy level; only has 1 orbital.
p sublevel
'dumb-bell' shaped orbitals along the x, y and z axis at 90° to each other; has 3 orbitals.

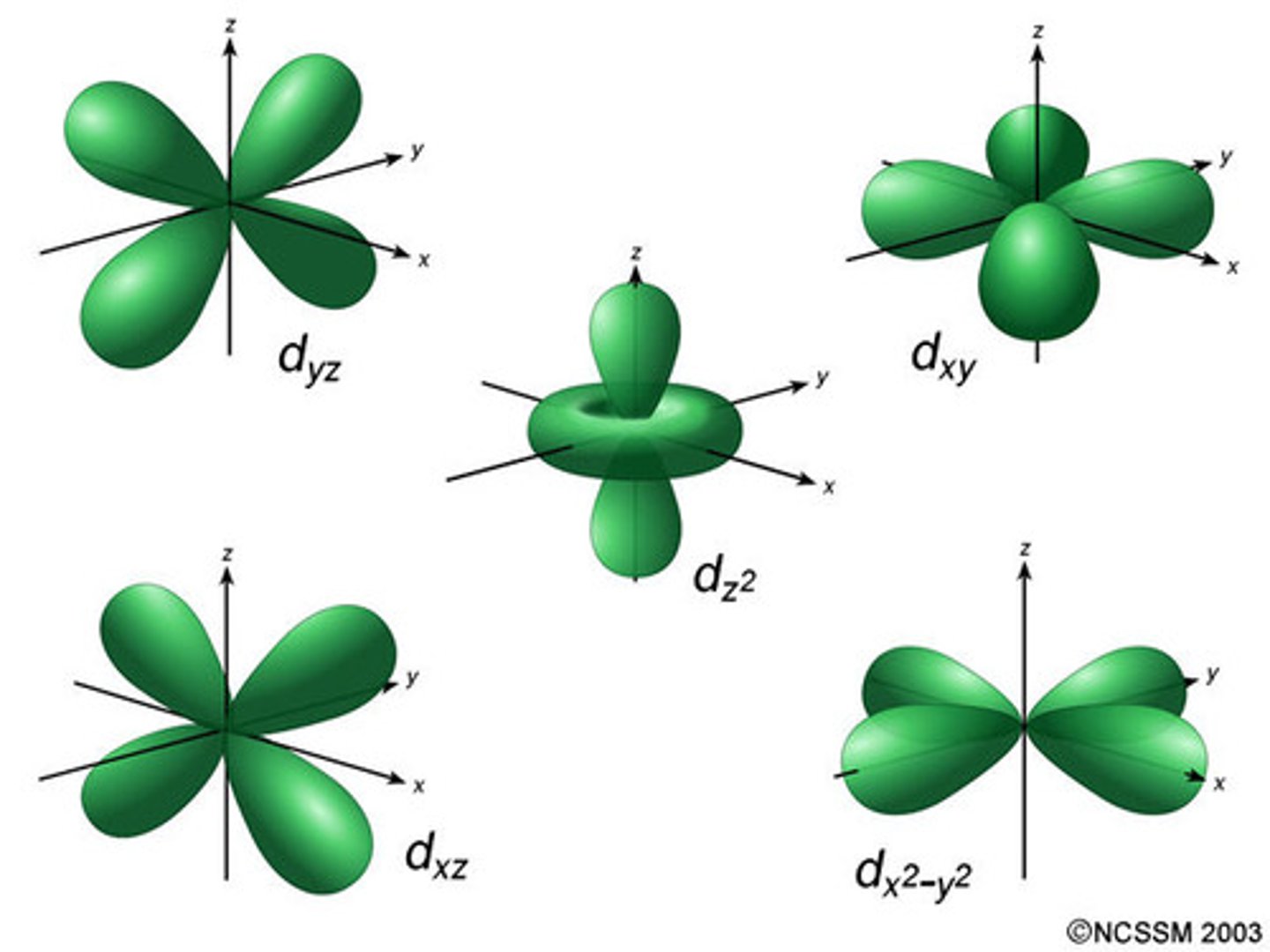
d orbitals
5 orbitals; third to fill for any energy level; first found in the third energy level
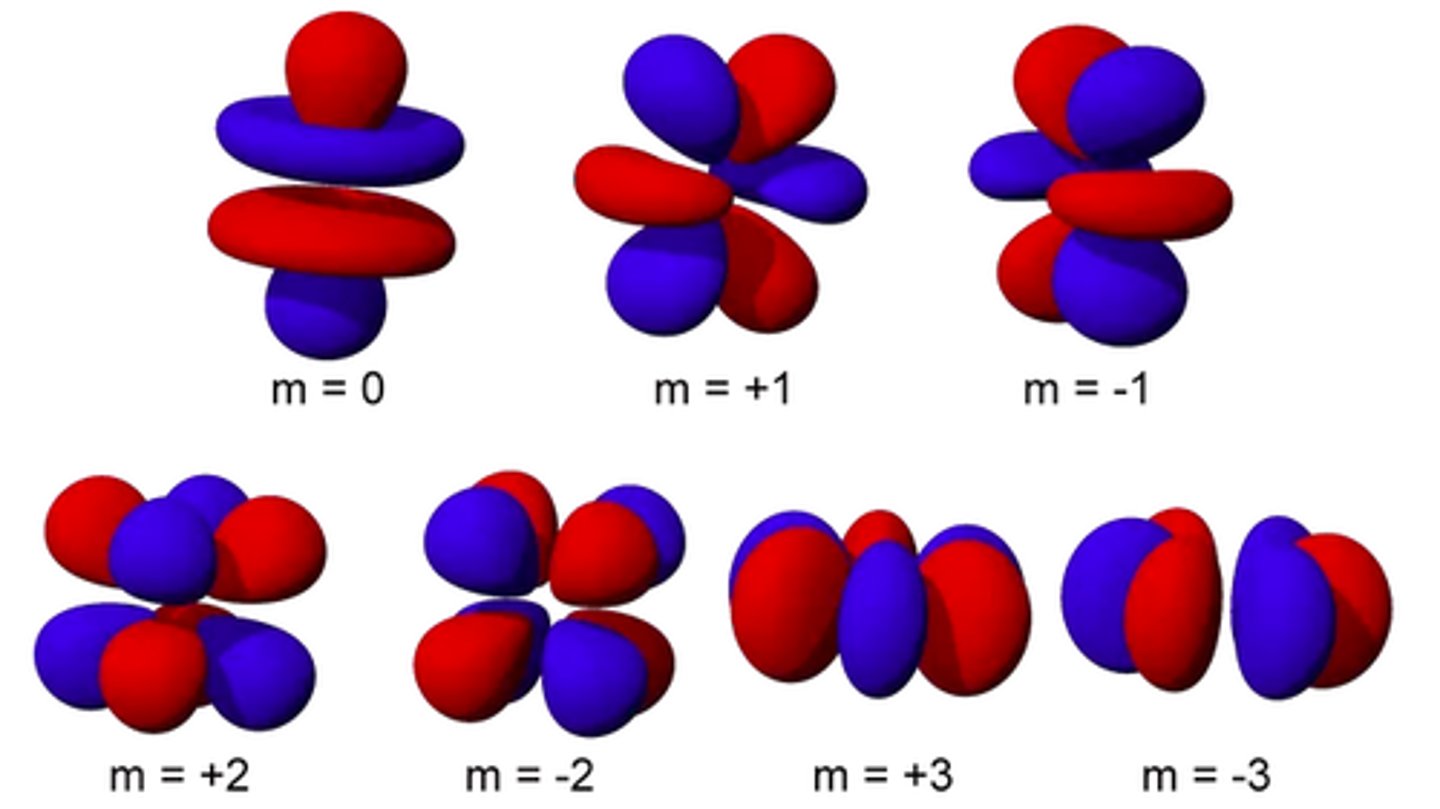
f orbitals
elements in the 2 rows below the main periodic table have electrons occupying these; has 7 orbitals
principal energy levels
correspond to:
- orbits in the Bohr model
-rows in the periodic table for the first 3 rows.
-leading number in the electron configuration
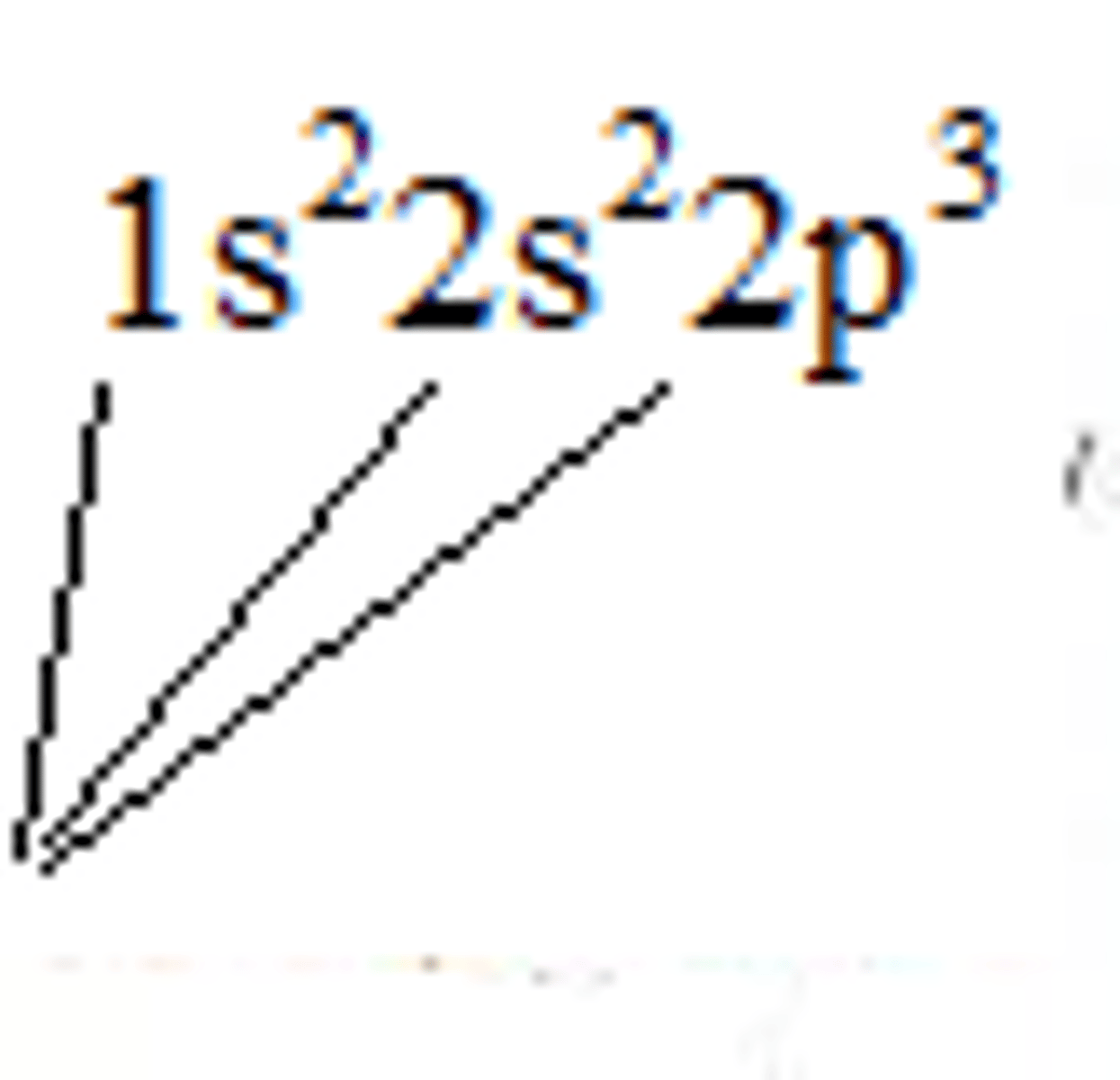
sublevels
correspond to
-what do the letter symbols stand for in the electron configuration (s, p, d, and f)
-splitting of the principal electrons levels because of electron repulsion
-columns in the periodic table
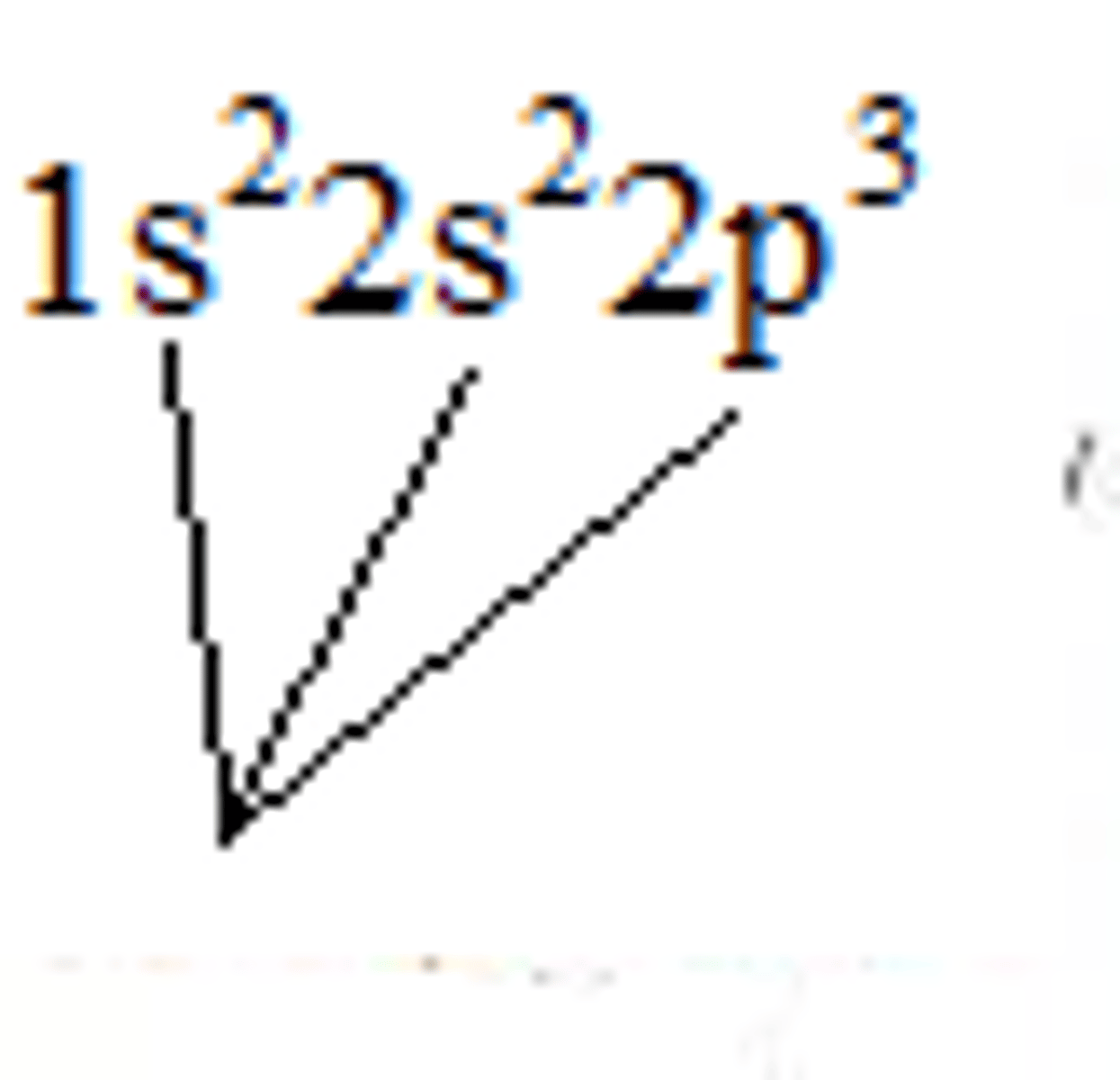
The number of electrons in a sublevel
what do the superscripts stand for?
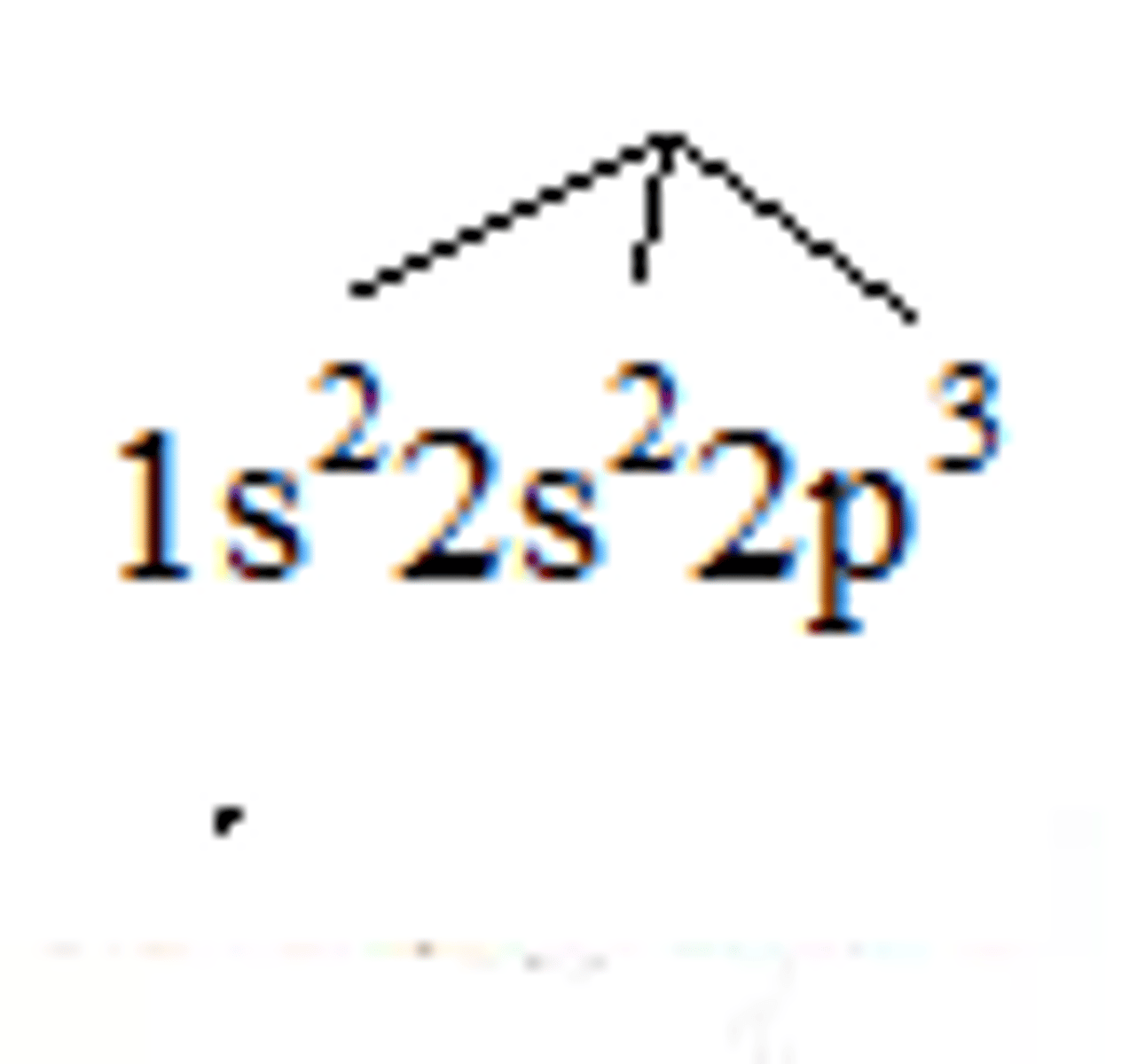
Nitrogen electron configuration
1s²2s²2p³

valence electrons
electrons in the highest occupied energy level of an atom; available for bonding

# of orbitals in any s sublevel
1
# of orbitals in any p sublevel
3
# of orbitals in any d sublevel
5
# of orbitals in any f sublevel
7
How do you determine the # of valence electrons using the electron configuration?
by counting the # of s and p electrons in the highest energy level.
Why do we fill the 4s orbital before the 3d orbital? (ENH)
because the 4s is lower in energy than the 3d, so it is filled first.
violet
in the visible light spectrum, which color has the highest frequency?
red
in the visible light spectrum, which color has the lowest frequency?
it decreases
As the wavelength gets longer, what happens to the energy?
How many valence electrons does this atom have? 1s²2s²2p⁶3s²3p²
4
ionization energy
the energy to remove an electron from an atom
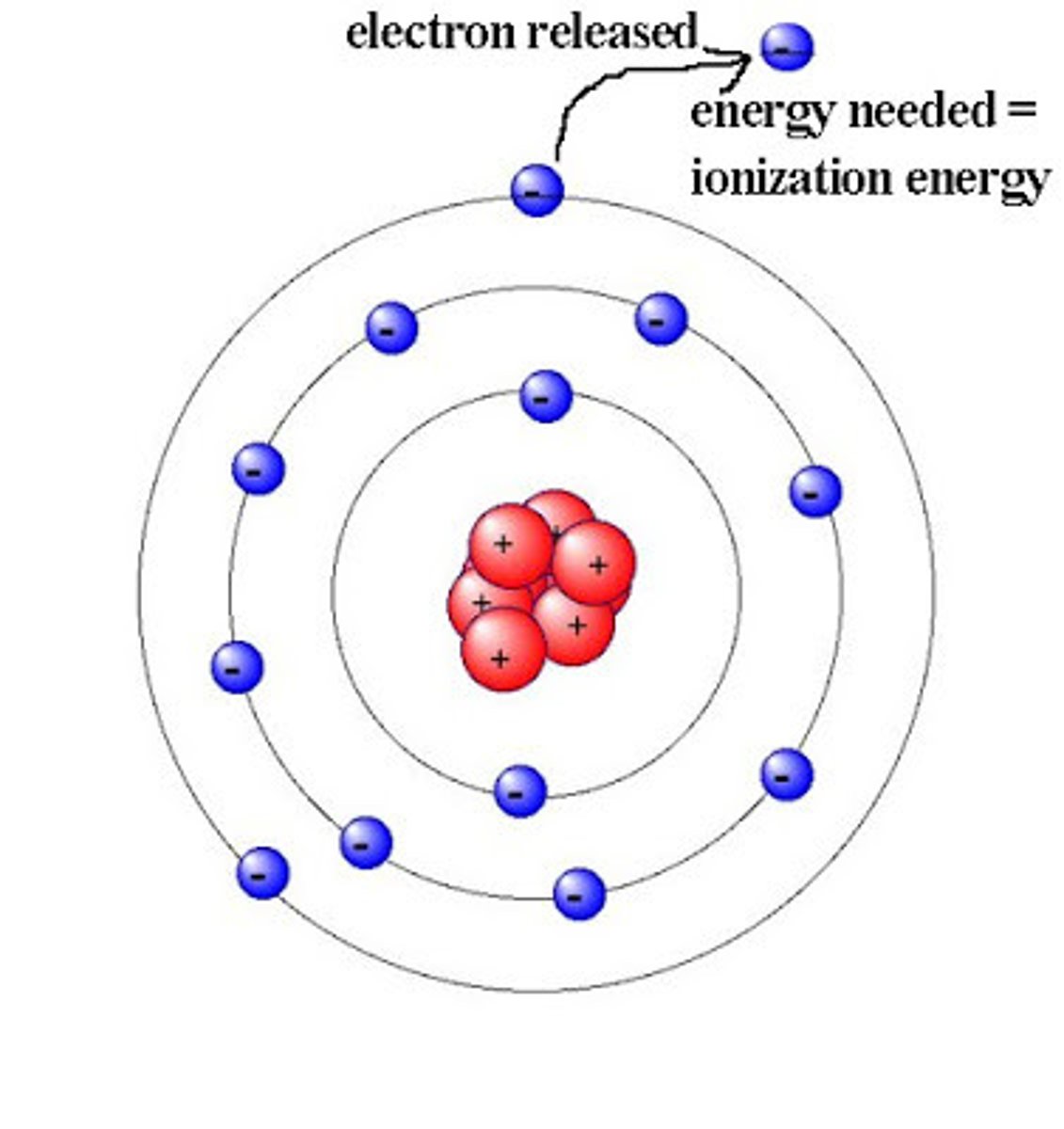
Bohr model of hydrogen
-fixed orbits
-moving between orbits requires an increase or decrease in potential energy
-energy is determined by coulombic attraction between electron and the nucleus
-explains the spectrum of hydrogen
Quantum model
- probability of electrons being in orbitals
- energy associated with orbitals is an ionization energy
-orbitals accommodate two electrons
-orbital fill from lowest energy level to highest
-electrons pair up when energy level is more than half full
absorption
light energy is used to move electron from a lower energy level to a high energy level. In the Bohr model, the electron moves away from the nucleus.
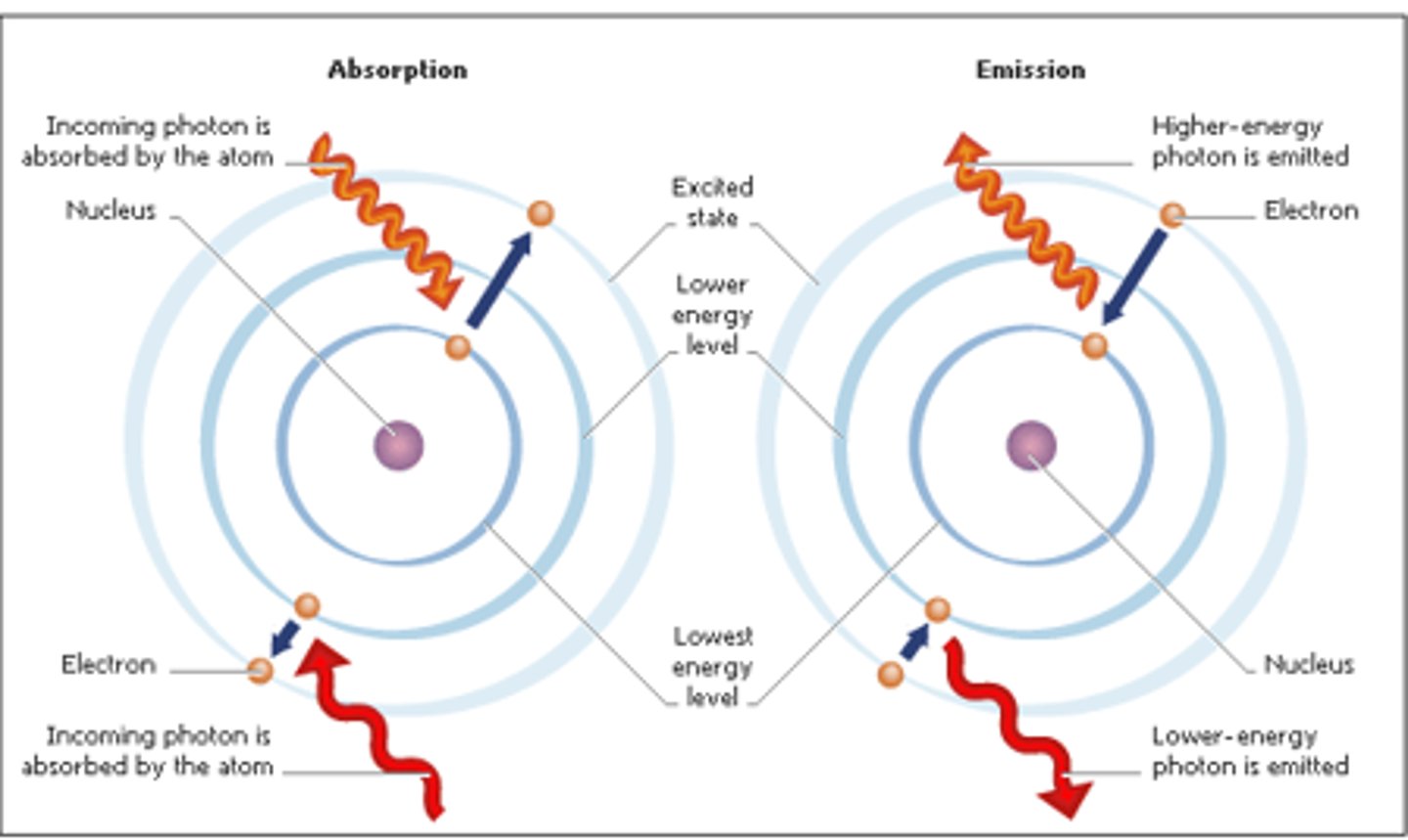
emission
energy is released when an electron moves closer to the nucleus

fluorescence
- atom absorbs energy of a higher energy such as UV and releases photons of lower energy such as visible light

phosphorescence
- a very slow form of fluorescence. Atom absorbs photons of high energy and very slowly releases photons of lower energy
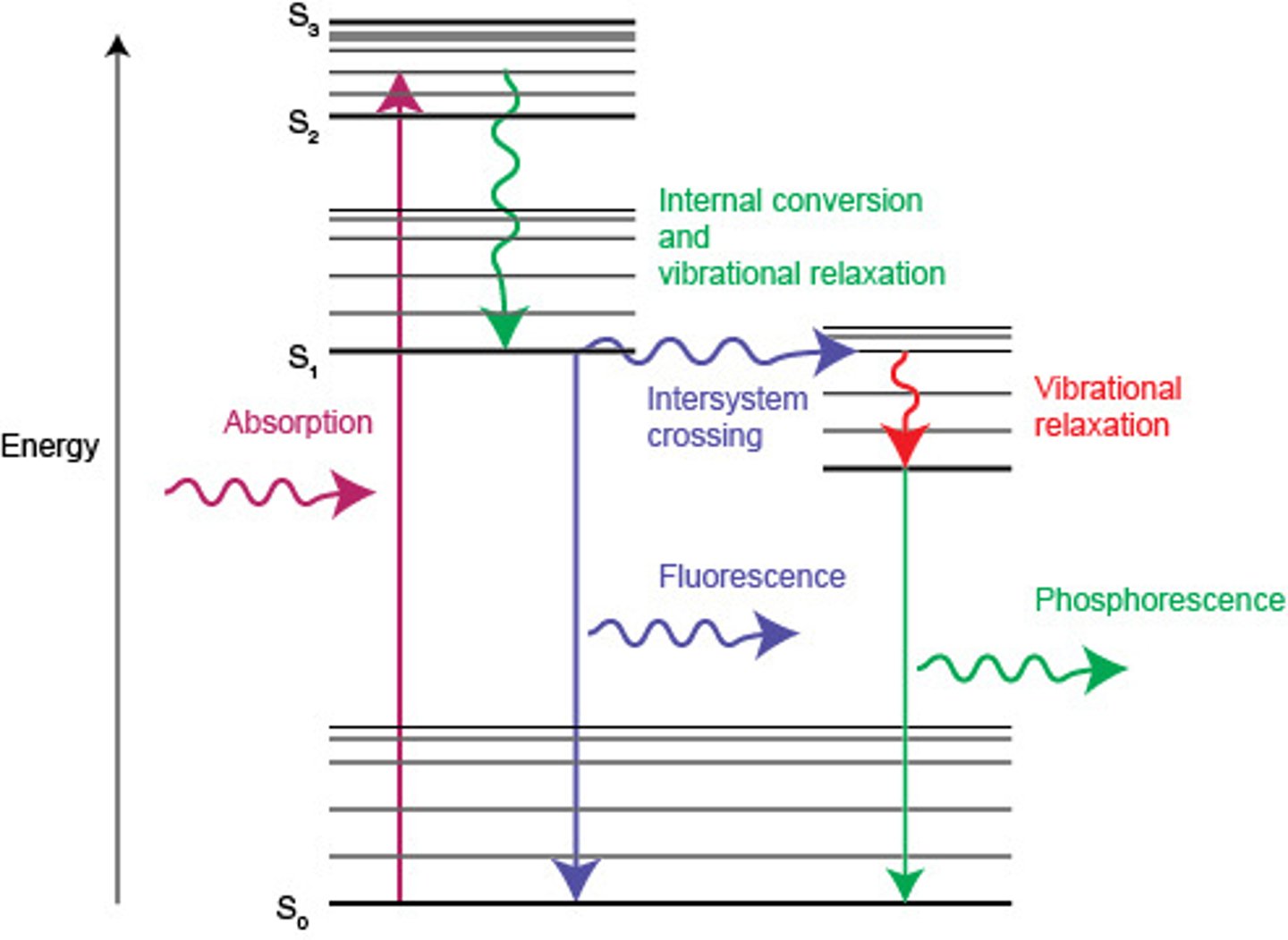
Ground state
an electron configuration in which all the electrons are in the lowest possible energy levels
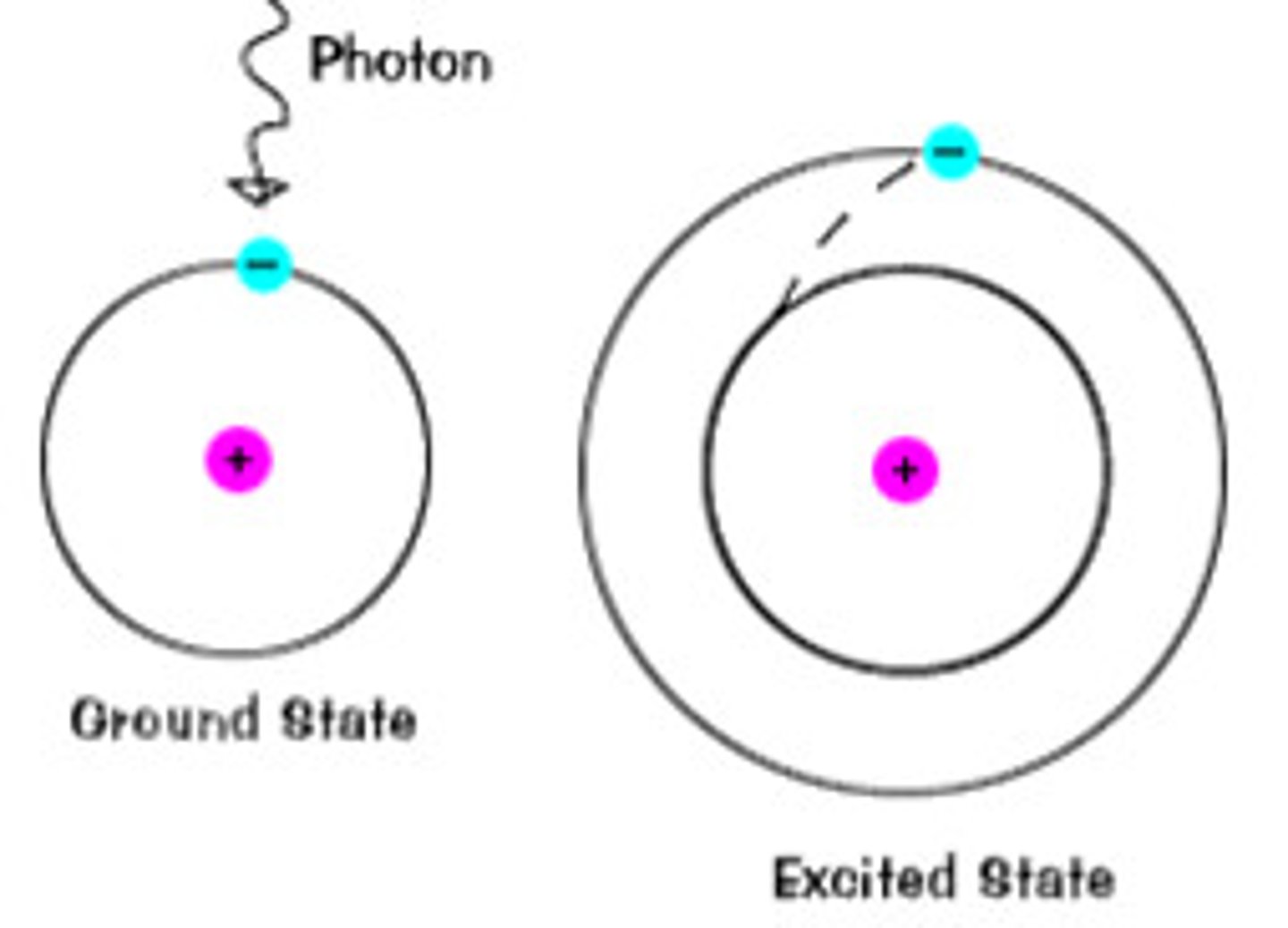
Excited state
electron configuration for an atom which has absorbed energy so that one or more electrons on in energy levels higher than the energy levels occupied in the ground state

Core electrons
electrons in filled energy levels
ion
an atom in which electrons have been added or removed so that the number of electrons does not equal the number of protons
determining the number of valence electrons using the periodic table
In the s and p blocks, count the number of periodic table squares from the left
s-block
columns 1 and 2 in the periodic table
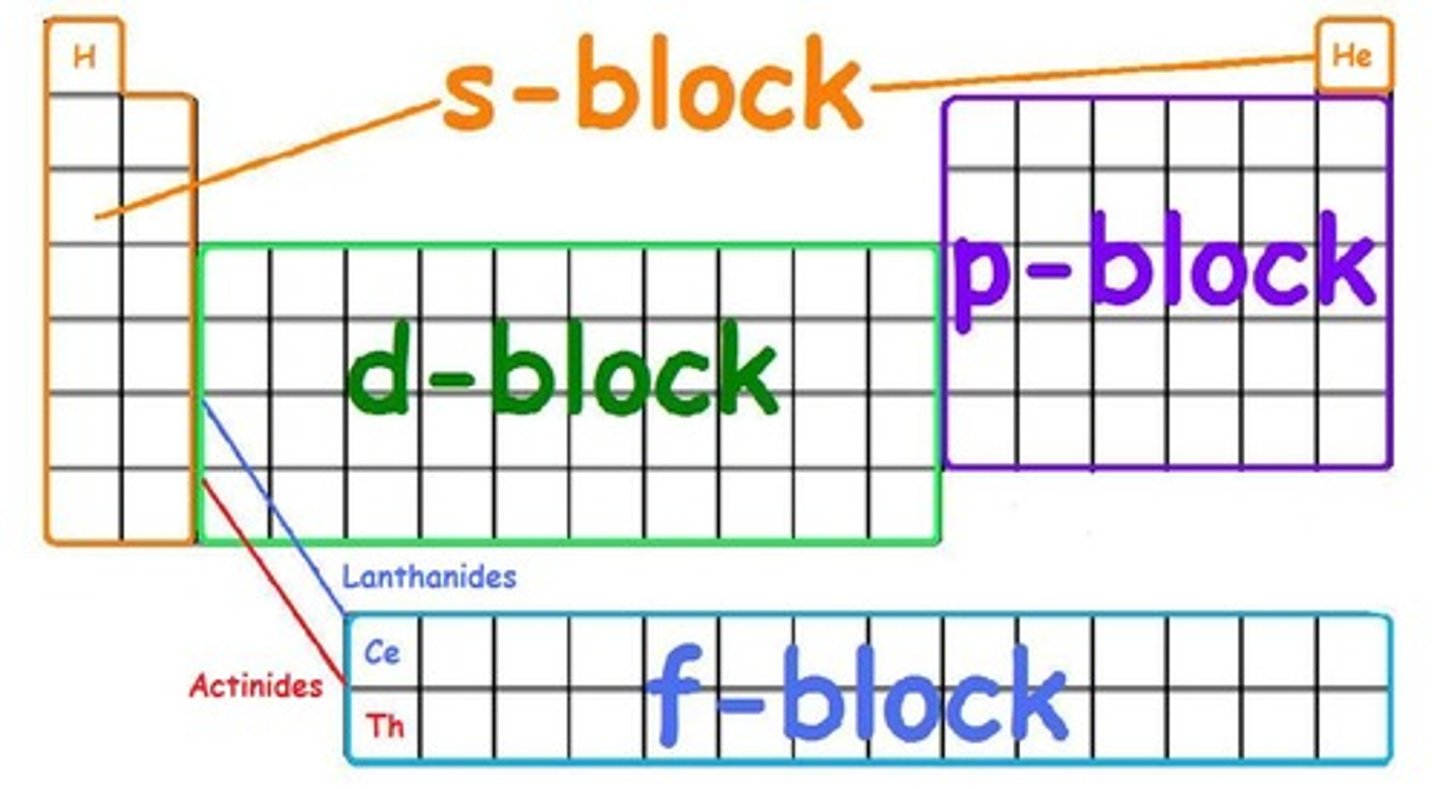
p-block
rightmost 6 columns in the periodic table

photon
a packet of light energy

wavelength
related to the size of a photon
hundreds of nanometers for visible light
Why does the quantum model of helium have sublevels?
repulsion between the two electrons
What is spin?
- quantity included in the quantum model to explain magnetism
-two possible states (up and down)
-think of electrons as acting like extremely tiny magnets
How do you figure out the total number of electrons in a neutral atom?
-atomic number
-add the superscripts of the electron configuration
photoelectric effect
when light energy absorbed is greater than the ionization energy of the valence electrons
quantum model explains
- chemical properties (valance electrons)
- magnetism ( up and down spin)
- absorption and emission spectra of elements (energy levels and sublevels)
Chemiluminescence
occurs when a product of a chemical reaction absorbs energy produced by the chemical reaction and emits light