16-Adrenal Glands
1/74
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
75 Terms
Adrenal Glands
These are located in the retroperitoneal cavity above each kidney and the hormone secretions are essential for life
The adrenal cortex and adrenal medulla are two separate glands that have different origins
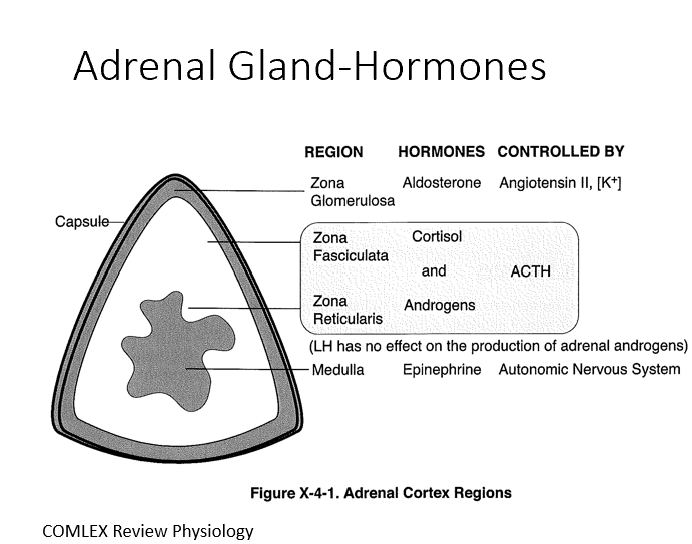
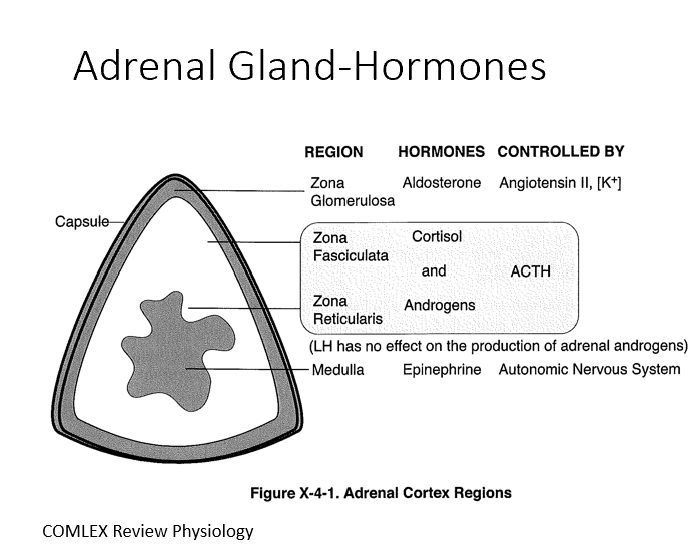
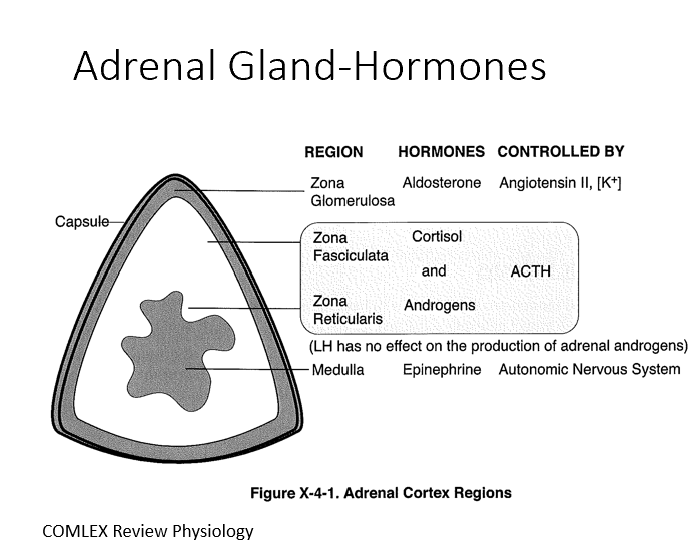
Adrenal Cortex
Mesodermal origin with three distinct layers (80% of tissue)
3 Layers:
Zona Glomerulosa (Aldosterone by ANG II)
Zona Fasciculata (Cortisol by ACTH)
Zona Reticularis (Androgens by ACTH)
Adrenal Medulla
Neuroectodermal origin and represents 20% of the tissue
Makes Epi by ANS (sometimes NE too)
Blood Supply for Adrenal Glands
Superior, middle, and inferior suprarenal arteries
Branches from the arteries form a network of capillaries that flow from the adrenal cortex to the medulla
Directionality in blood flow results in a high concentration of steroid hormones being delivered to the adrenal medulla
Cortisol influences the biosynthetic pathway of catecholamines
Venous Drainage Adrenal Glands
Venous drainage is via a single renal vein on each side (right→inferior vena cava and left→left renal artery)
Synthesis of Catecholamines (based on, controlled by, released by)
Based on a tyrosine derivative!
Under control of the CRH-ACTH-cortisol axis
ACTH stimulates synthesis of DOPA
Cortisol increases PNMT (upregulates NE→Epi)
Release is triggered by ANS control
Neurons and Adrenal Medulla
Synthesis of catecholamines happening in these 2 locations:
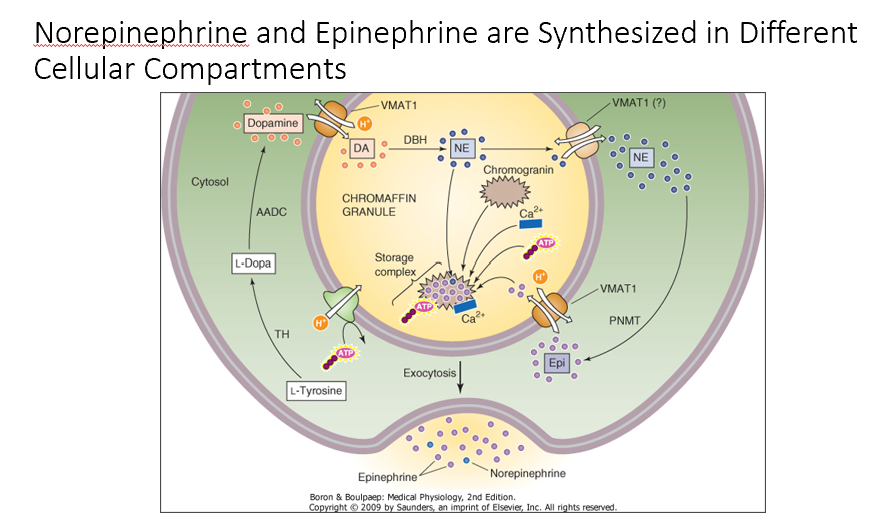
Norepinephrine and Epinephrine
THESE are Synthesized in Different Cellular Compartments
NE in chromatin granules
Once in cytosol, PMNT can act on NE to make Epi!
Catecholamine Receptors
Adrenergic receptors
Types:
Alpha 1
Alpha 2
Beta 1
Beta 2
Beta 3
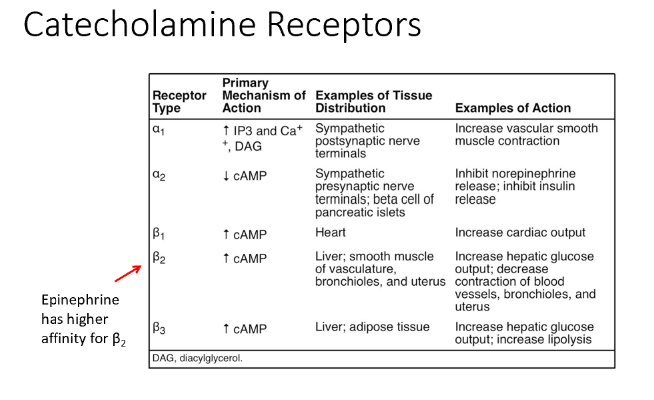
Alpha 1 Receptor
Tissue Distribution: Sympathetic postsynaptic nerve terminals
Action: Increase vascular smooth muscle contraction
Alpha 2 Receptor
Tissue Distribution: Sympathetic presynaptic nerve terminals; beta cells of pancreatic islets
Action: Inhibit norepinephrine release; inhibit insulin release
Beta 1 Receptor
Tissue Distribution: Heart
Action: Increase cardiac output
Beta 2 Receptor
Tissue Distribution: Liver; smooth muscle of vasculature, bronchioles, and uterus
Action: Increase hepatic glucose output; decrease contraction of blood vessels, bronchioles, and uterus
EPI has a higher affinity for this!
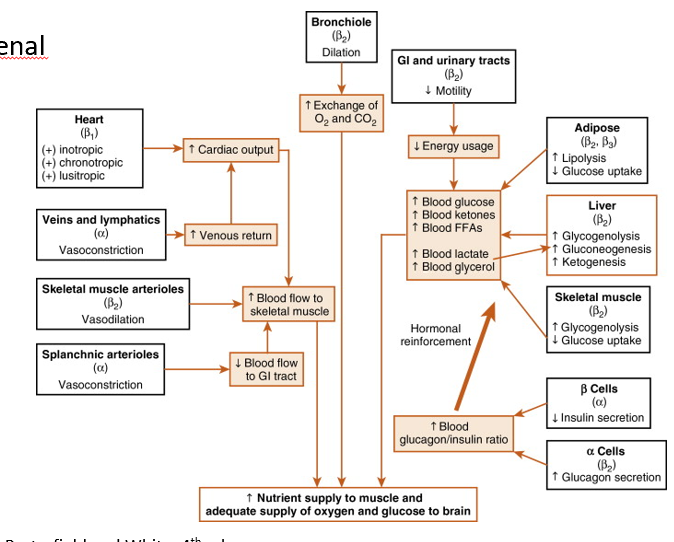
Integrated Catecholamine Response to Exercise
Increased CO, venous return, & blood flow to skel muscle
Alpha receptors simultaneously decrease blood flow to GI
Bronchioles (via Beta 2 receptors) dilate!
Increases exchange of O2 and CO2
Overall, Catecholamines stimulate increase in blood glucose and mobilization of other energy stores (increasing nutrient supply to mm.)
lipolysis
gluconeogenesis
ketogenesis
decreasing glucose uptake
Beta Cells- decrease in insulin secretion
Alpha Cells- increase in glucagon secretion
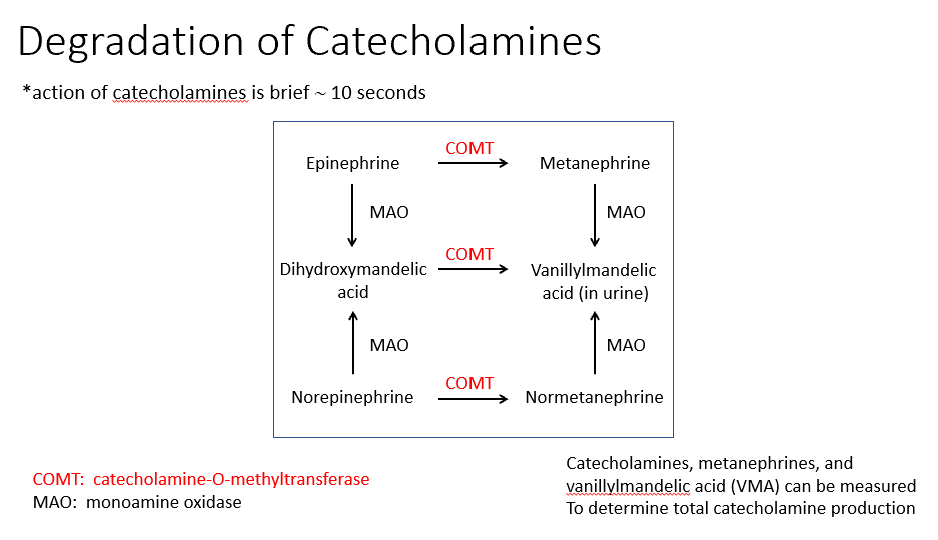
Degradation of Catecholamines
They’re only active for 10 seconds
Degradation happens via 2 enzymes:
COMT: catecholamine-O-methyltransferase
MAO: monoamine oxidase
At the end of degradation of NE/Epi, we see Metanephrine 7 Vanillymandelic Acid (found in urine).
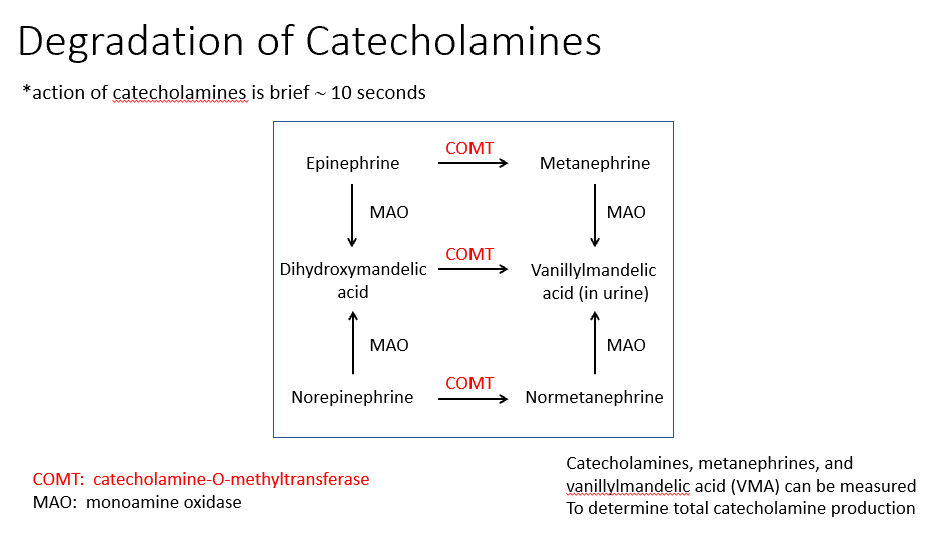
Determining total catecholamine production
Catecholamines, metanephrines, and
vanillylmandelic acid (VMA) can be measured
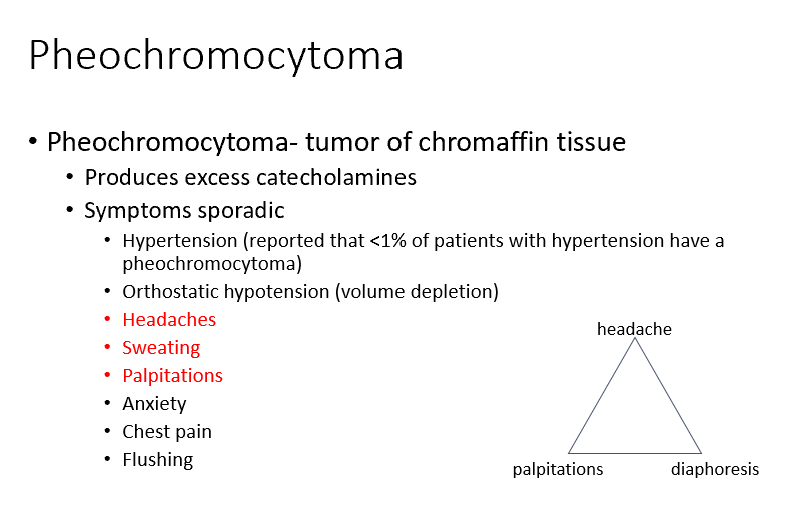
Pheochromocytoma
Tumor of chromaffin tissue
Produces excess catecholamines
Symptoms sporadic
Headaches
Sweating
Palpitations
Adrenal Cortex Hormones
Aldosterone
Cortisol
Androgens
Aldosterone
(mineralocorticoid- regulates salt and water retention)
Functions in salt and water homeostasis
Cortisol
(glucocorticoid- increases plasma glucose)
Released in response to stress
Influences glucose utilization, immune and inflammatory homeostasis
Androgens
dehydroepiandrosterone (DHEA)
Primary androgenic hormone in females
Biosynthetic Pathway for Adrenal Hormones: Enzymes
We start out with cholesterol and then numerous enzymes are involved in taking that cholesterol and turning it into adrenal hormones:
17-Alpha Hydroxylase
21-Alpha Hydroxylase
11-Beta Hydroxylase
Aldosterone Synthase
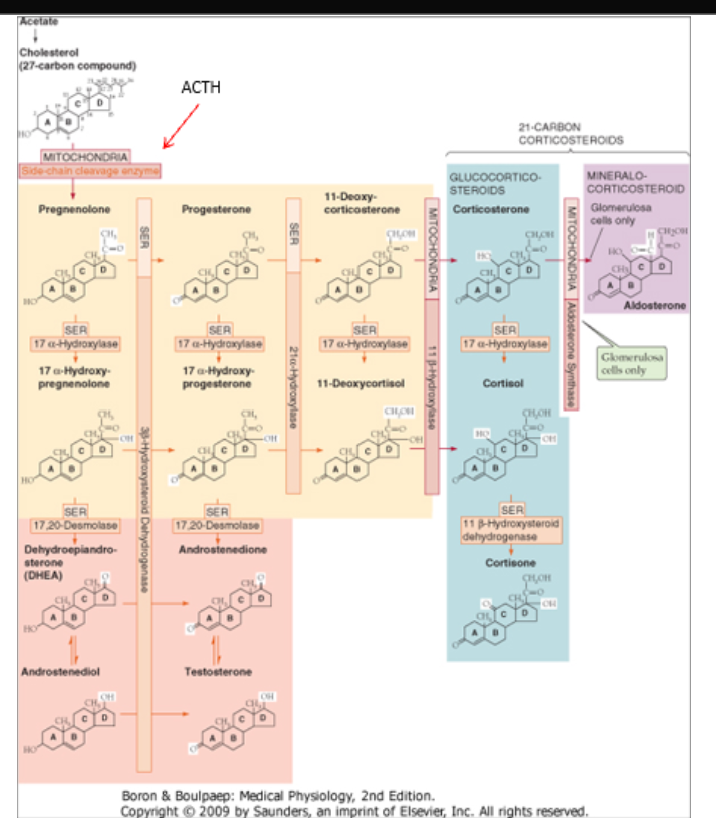
Cholesterol
What all adrenal hormones are derivatives of
That Zone
Each zone of the adrenal cortex contains a specific set of enzymes that controls hormone production for _.
Aldosterone
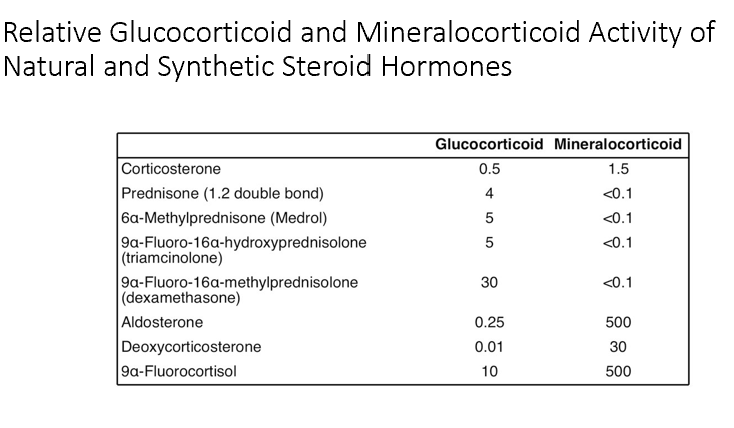
Converts glucocorticoids into mineralocorticoids in the biosynthetic pathway.
Gluc and Mineral corticoids can have overlapping activity on the receptors of each particular hormone.
Thus, our body has put only Gluc or Mineral corticoid receptors in specific areas so it only receives the one it’s supposed to.
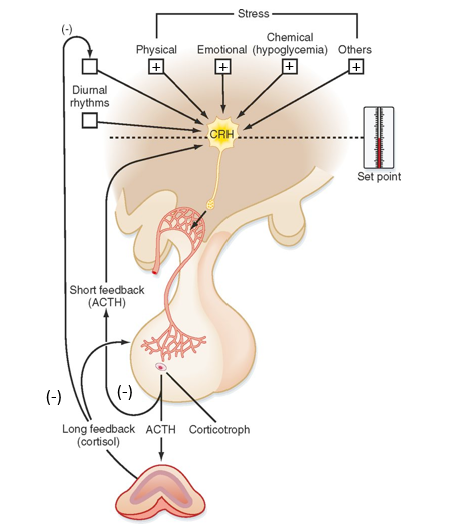
Cortisol (what is it, stimulating factors, inhibiting factors, pathway)
Hormone from the adrenal cortex:
Stimulatory Factors:
Stress
Sleep-wake transition
Decreased blood cortisol levels
Inhibitory Factors:
Increased blood cortisol levels (it inhibits itself)
Somatostatin
Pathway:
Stress helps regulate CRH from hypothalamus
CRH stimulates ACTH production in pituitary gland
When ACTH is release, it can work on adrenal cortex to induce synthesis of glucocorticoids/cortisol
Cortisol feedback loops:
Negative- inhibits ACTH & CRH
Actions of Cortisol
Increases gluconeogenesis, protein catabolism, lipolysis, and decreases glucose utilization and insulin sensitivity
Anti-inflammatory effects
Suppresses immune responses
T cell suppression (IL-2)
Lyses eosinophils
Maintain vascular responsiveness to catecholamines
Maintains normal blood pressure
↓ cortisol → hypotension
Inhibition of bone formation
Decreases type I collagen
Decreases osteoblast activity
Decreases gut Ca2+ absorption
Increases glomerular filtration rate (GFR)
Decreases REM sleep (psychosis)
Acute Release of Cortisol
Cortisol Increases
Insulin/Glucagon Ratio Decreases
Epi and NE Increases
Using our energy stores, increase in glucose production
Metabolic response to stress ensures there’s enough energy to meet increased demands (aka enough glucose for brain).
Cortisol is increasing energy
Cortisol is limiting immune response to stress
Chronic Release of Cortisol
Cortisol Increased
Insulin/Glucagon Ratio Increased
Epi & NE Decreased
Increased appetite, increased glycogen synthesis, increased protein synthesis, decreased GLUT-4 glucose intake (Cortisol is antagonizing insulin)! Decreased lipolysis and increased TAG synthesis.
Metabolic responses: localized obesity & muscle weakening. Decreased glucose uptake due to cortisol antagonizing insulin. See both hyperglycemia and hyperinsulinemia.
Steroid Cushing Syndrome
overreplacing glucocorticoids/cortisol to treat their chronic inflammatory condition → Cushing Syndrome (too much cortisol) and Adrenal Atrophy (body isn’t making its own anymore)
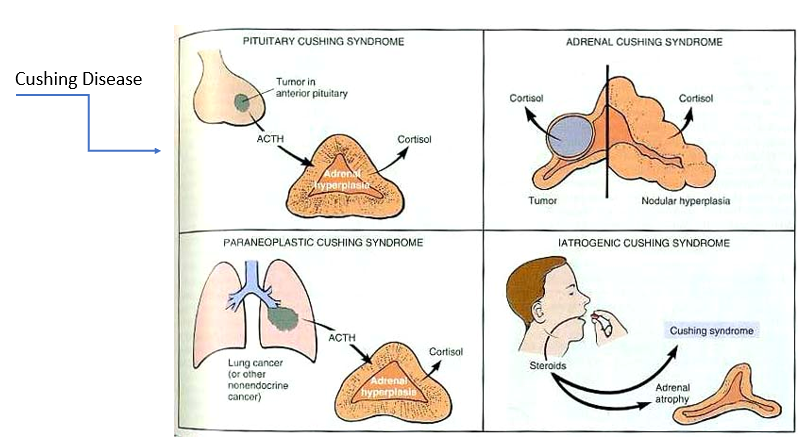
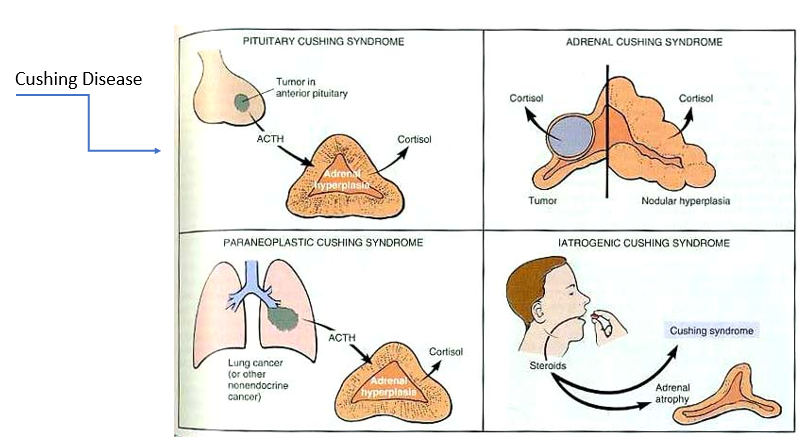
Cushing Disease
Tumor in anterior pituitary (overproducing ACTH) → too much cortisol → adrenal hyperplasia
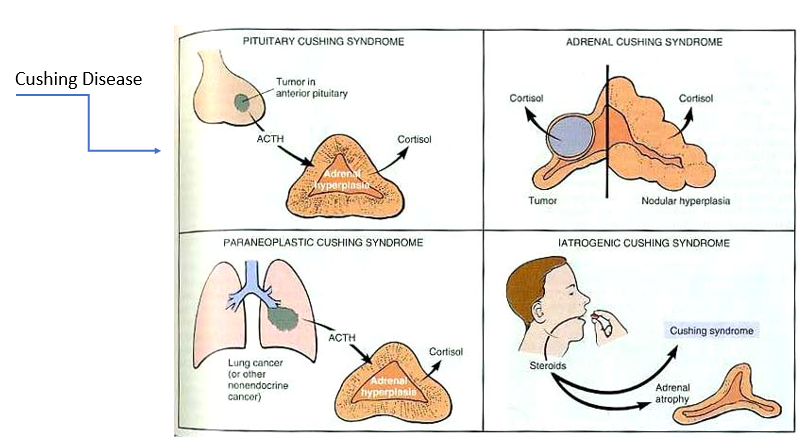
Adrenal Cushing Syndrome
Tumor in adrenal cortex or nodular hyperplasia → too much cortisol
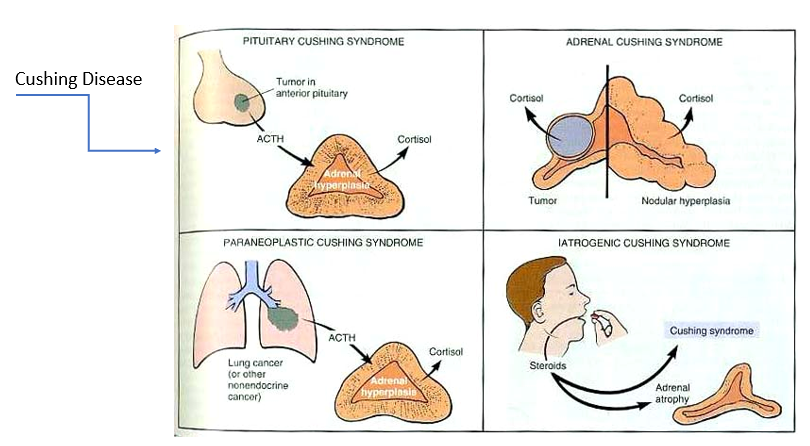
Paraneoplastic syndrome
tumor in location other than anterior pituitary that’s overproducing ACTH → too much cortisol
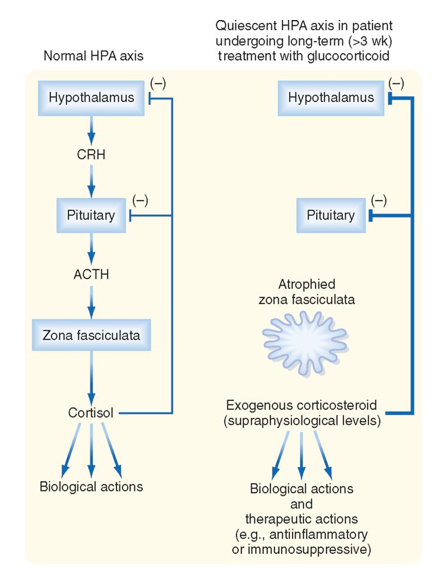
Why do we see Adrenal Atrophy in the Presence of Exogenous Glucocorticoids?
Normal:
Hypothalamus → CRH → Pituitary → ACTH → Zona Fasciculata → Cortisol
Too many exogenous glucocorticoids:
Too much corticosteroid present → feeds back to inhibit CRH from hypothalamus & ACTH from pituitary
No stimulation of hypothalamus & pituitary = no hormones produced (CRH and ACTH) = Zona Fasciculata atrophies
This is why we have taper doses of steroids, to allow adrenal gland to build back up and make its own again!
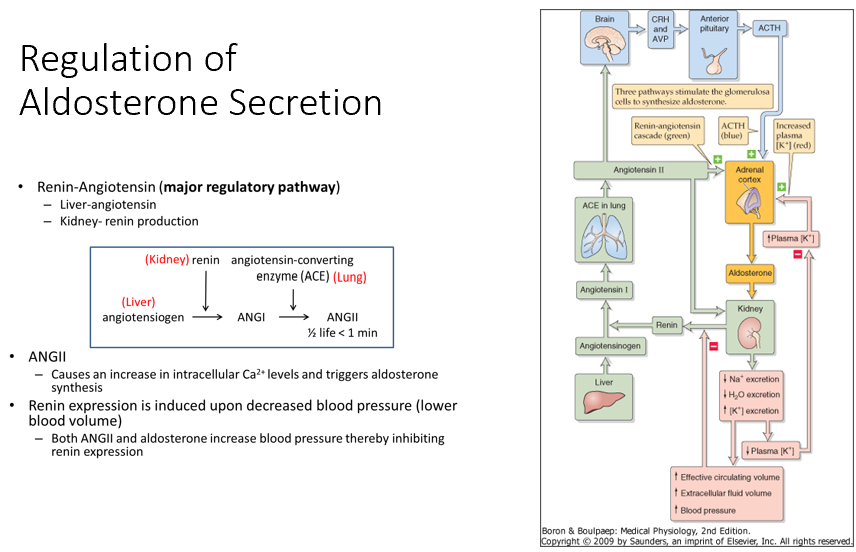
Aldosterone
Renin-Angiotensin (Major Regulatory Pathway)
Liver: Angiotensinogen
Kidney: Renin production
Pathway
Angiotensinogen (Liver) → via Renin (in kidney)→ Angiotensin I (ANGI) → via ACE (in lung)→ Angiotensin II (ANGII)
ANGII
Triggers aldosterone synthesis by acting on adrenal cortex
Aldosterone then works on our kidneys to decrease Na/Water excretion (but increases K secretion)
Renin Expression
Induced by decreased blood pressure (lower blood volume)
When BP increases (thanks to aldosterone), it shuts renin down!
Note: High K levels will promote synthesis of aldosterone & decrease K retention!
Actions of Aldosterone (what it controls, vs ADH, receptors, lack, excess)
Controls Na+ resorption in the extracellular space
Increases Na+ resorption, increases K+ and H+ secretion
Contrast THIS to ADH which regulates water balance and hence plasma osmolality
ADH doesn’t impact Na absorption, THIS does
Receptors
Kidney- target cells have 11β-HSD2 (11B-hydroxysteroid dehydrogenase II) that converts cortisol to cortisone which has low affinity for the mineralocorticoid receptor (MR)
Colon
Salivary glands
Sweat glands
Lack of aldosterone
Hyperkalemia, hypotension, metabolic acidosis
Excess aldosterone
Hypokalemia, hypertension, metabolic alkalosis
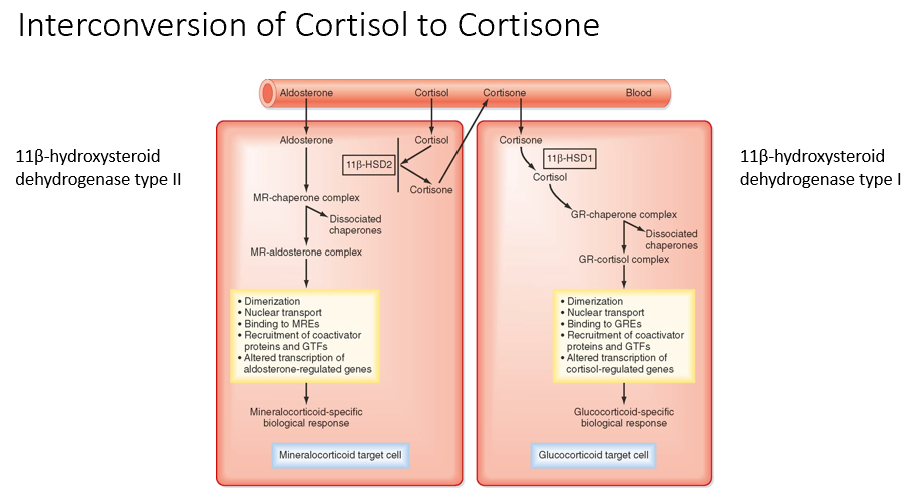
Interconversion of Cortisol to Cortisone
11β-hydroxysteroid dehydrogenase type II is for mineralocorticoid cells (Cortisol → Cortisone)
11β-hydroxysteroid dehydrogenase type I is for glucocorticoid cells (Cortisone → Cortisol)
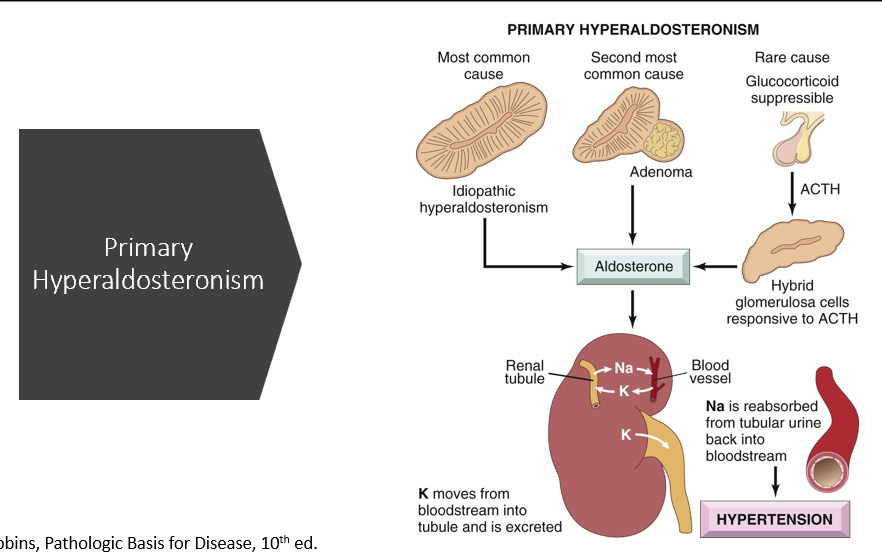
Primary Hyperaldosteronism (causes & what happens)
Most common cause:
Idiopathic (generalized increase in the zona glomerulus)
Second most common cause:
Adenoma in adrenal cortex
What happens:
too much aldosterone
Na is reabsorbed, water follows (develops HTN)
K is excreted (hypokalemia)
H loss (metabolic alkalosis)
Actions of Adrenal Androgens
Females
Presence of pubic and axillary hair, libido
In females the only primary of androgens is from the adrenal cortex
Males
Same as testosterone
Men do not need adrenal androgens as they are synthesized in the testes
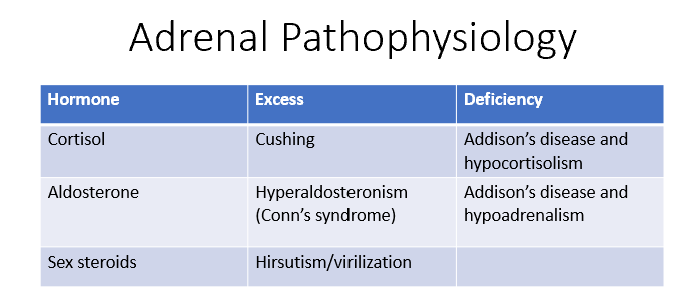
Adrenal Pathophysiology
Cortisol:
Excess = Cushing's syndrome
Deficiency = Addison's disease and hypocortisolism
Aldosterone:
Excess = Hyperaldosteronism (Conn's syndrome)
Deficiency = Addison's disease and hypoadrenalism.
Sex steroids:
Excess = Hirsutism or virilization.
Congenital Adrenal Hyperplasia
A group of autosomal recessive disorders that involve a deficiency in either cortisol, aldosterone, or both due to impaired synthesis.
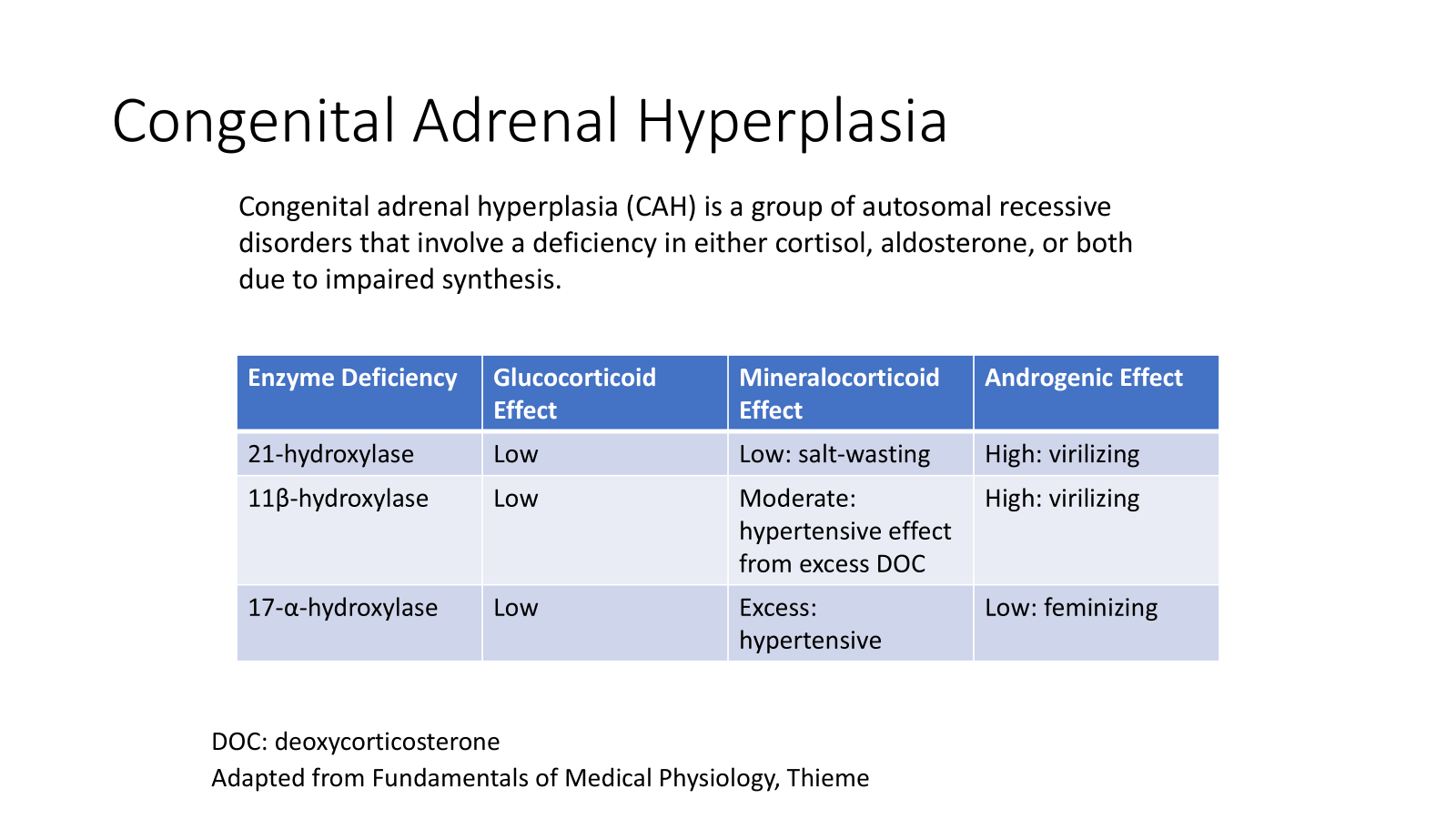
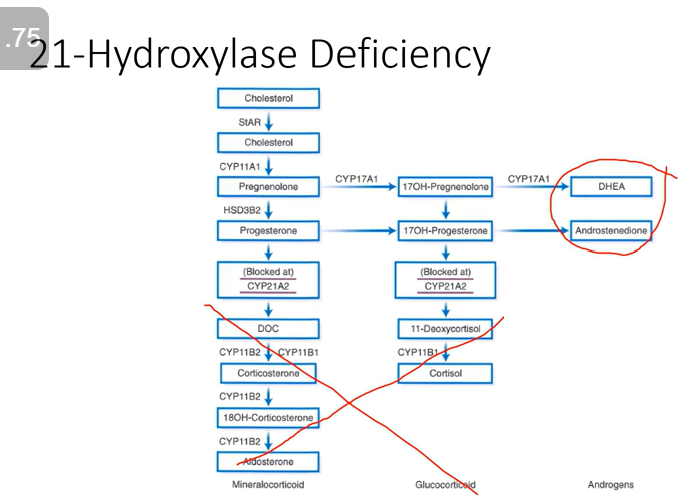
Enzyme Deficiency: 21-hydroxylase
Glucocorticoid Effect: Low
Mineralocorticoid Effect: Low; salt-wasting
Androgenic Effect: High; virilizing
Buildup of precursors but we DON’T get cortisol or any other corticosteroids. We’re left with excess androgens.
Caused by:
Mutation in CYP21A2 gene!
Can have total loss or partial loss
Total loss:
Salt-wasting
Hyponatremia
Hyperkalemia
Hypotension
Cardiovascular collapse and possibly death
Virilization- recognizable in females at birth but more difficult in males
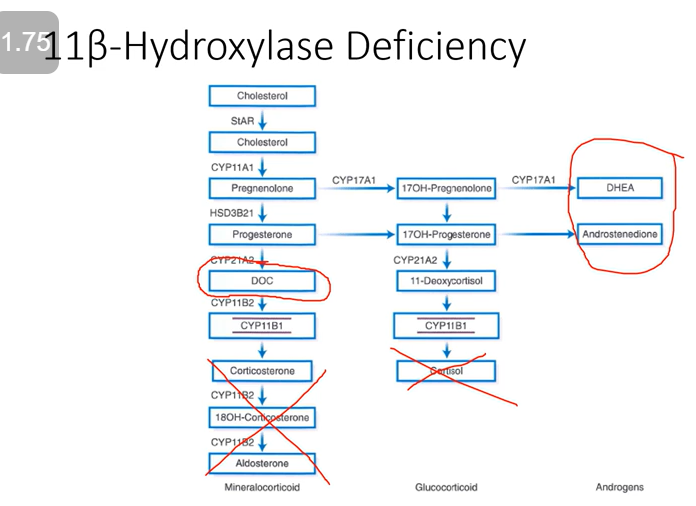
Enzyme Deficiency: 11β-hydroxylase
Glucocorticoid Effect: Low
Mineralocorticoid Effect: Moderate; hypertensive effect from excess deoxycorticosterone (DOC)
Androgenic Effect: High; virilizing
We lose cortisol and aldosterone BUT deoxycorticosterone is produced (it has some mineralocorticoid activity). We still produce our androgens!
Caused by:
Gene mutation in CYP11B1
Loss of negative feedback inhibition and ACTH-mediated adrenal androgen excess is observed
Mineralocorticoid activity is attributed to deoxycorticosterone (DOC) and increased secretion is postulated to be responsible for the observed hypertension in these patients
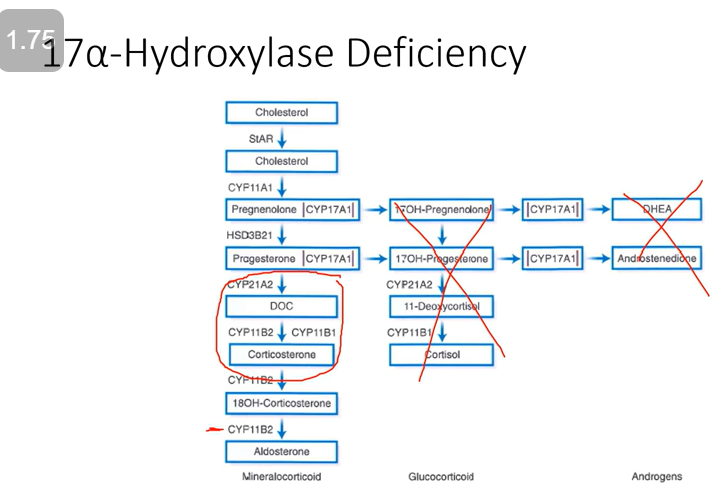
Enzyme Deficiency: 17-α-hydroxylase
Glucocorticoid Effect: Low
Mineralocorticoid Effect: Excess; hypertensive
Androgenic Effect: Low; feminizing
ACTH will be high, cortisol is gone, and we’ve lost adrenal androgens. BUT we have a build-up of DOC (thus overload of mineralocorticoids).
Caused by:
Mutation in gene CYP17
Impaired sex steroid and cortisol biosynthesis
Excess intermediary steroids with mineralocorticoid activity leads to varying degrees of hypertension and hypokalemia
No androgen activity
Females fail to develop secondary sexual characteristics
Males develop ambiguous external genitalia
During exercise, epinephrine and norepinephrine act to:
a. Increase muscle glycogenolysis
b. Increase hepatic glycolysis
c. Decrease adipocyte lipolysis
d. Decrease hepatic ketogenesis
a. Increase muscle glycogenolysis
A congenital null mutation of 11- hydroxylase would result in:
a. Sodium wasting
b. Hyperglycemia
c. Masculinization of a female fetus
d. Atrophy of adrenal cortex
c. Masculinization of a female fetus
One treatment for hypertension is the use of aldosterone receptor antagonists. A side effect of this treatment can be:
a. Cardiac hypertrophy
b. Hyperkalemia
c. Metabolic alkalosis
d. Edema
b. Hyperkalemia
Glucocorticoid analogs are used at high levels to suppress inflammation. A side effect of this treatment can be:
a. Adrenocortical hypertrophy
b. Hypoglycemia
c. Hyperpigmentation of the skin
d. Osteoporosis
d. Osteoporosis
Cortisol is prevented from interacting with the mineralocorticoid receptor in the distal nephron through the action of:
a. Cortisol-binding protein
b. 11B-hydroxysteroid dehydrogenase type 2
c. 17B-hydroxysteroid dehydrogenase type 1
d. Serum and glucocorticoid-regulated kinase (SGK)
b. 11B-hydroxysteroid dehydrogenase type 2

What is Lily’s diagnosis?
Which of the following agents should be administered first to control Lily’s blood pressure prior to surgery?
Pheochromocytomas
An α1-adrenergic antagonist
(Don’t want to give Beta-adrenergic antagonist first because if someone is constricted, you run the risk of hypoperfusion and death of tissues.)


Predict how the following serum values will be altered in this case compared to normal based on a deficiency of 21-hydroxylase (Aldosterone, K, Na, Renin).
Aldosterone will be decreased
K will be increased
Na will be decreased
Renin will be increased


The most likely enzyme deficiency is:
17α-hydroxylase

Causes of Cushing’s Syndrome
ACTH-dependent
ACTH-independent
Factitious
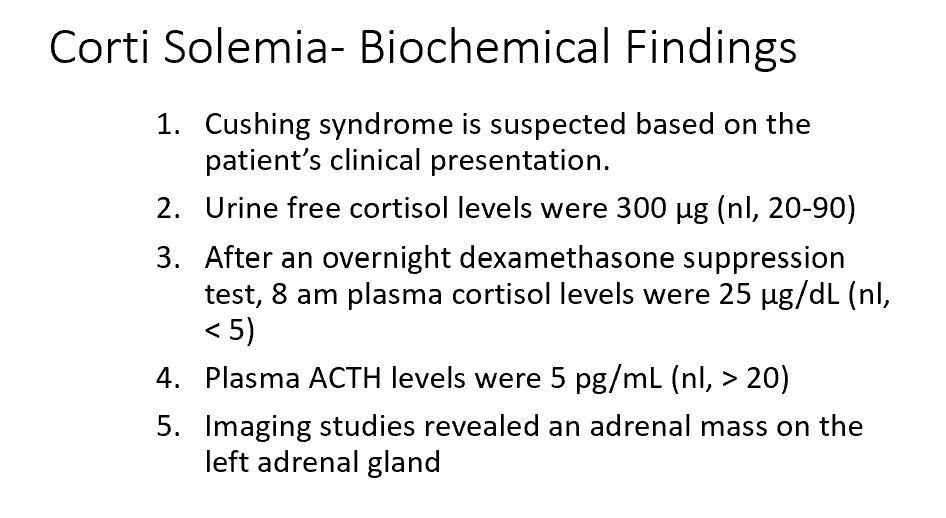
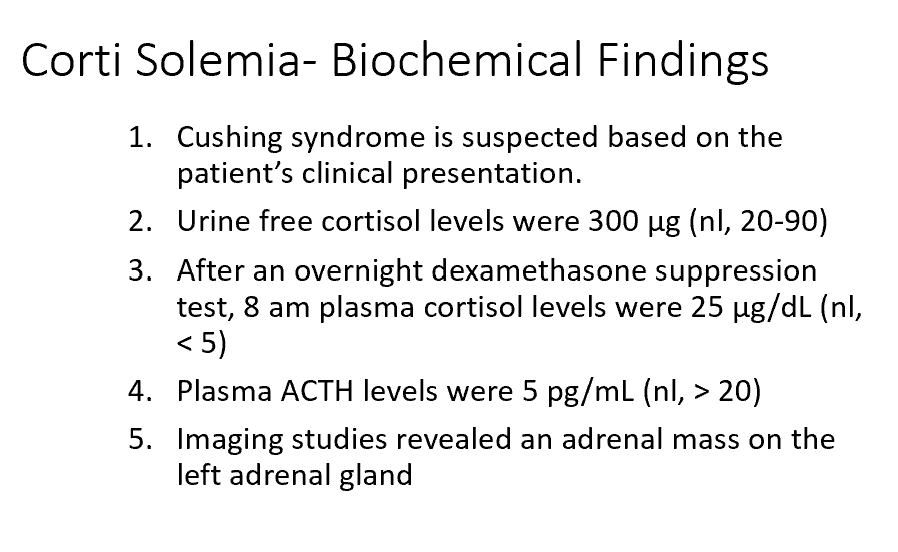
The results from Corti are most consistent with an overproduction of THIS from THIS:
Why was Corti’s response to the dexamethasone suppression test abnormal?
Cortisol from an adrenal tumor
There’s unregulated secretion of ACTH/Cortisol from the adrenal tumor

Corti had increased central obesity, muscle wasting, and striae. What are the biological effects of cortisol that are responsible for each of these physical findings?
Hyperlipidemia, lipolysis, adipose deposition changes, proteolysis too (muscle wasting).
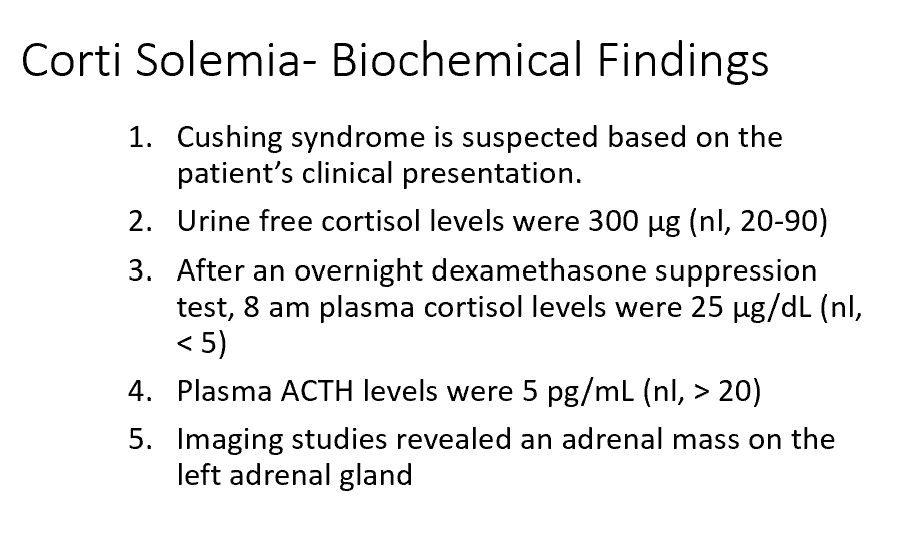
What might you expect to see upon a bone scan?
Corti’s blood pressure was 165/105. Explain the hypertension.
Why was Corti’s blood glucose 160 mg/dL?
Chronic = decrease in bone density
Cortisol increases cardiac output via Alpha 1 Receptors!
Increased Cortisol = decrease in glucose uptake and insulin secretion. Decrease in GLUT 4 receptor expression.
Some cases of marked hypercortisolemia are associated with a hypokalemic alkalosis. What is the physiologic basis for the hypokalemia in these cases?
Oversaturation of 11β-hydroxysteroid-dehydrogenase (11β-HSD2 enzyme).
(Found in mineralocorticoid receptor tissues like the kidney!
Oversaturation of this enzyme results in cross reaction of it with mineral and glucocorticoids.)
Corti had his left adrenal gland removed and was subsequently placed on exogenous glucocorticoids. What is the rationale for replacement of glucocorticoids at this time?
The pituitary corticotrophs have atrophied over the course of Corti’s disease.
(One adrenal gland is more than adequate!
What we see in this case is Corti has high cortisol levels, he’s not making CRH from hypothalamus or ACTH from pituitary.)
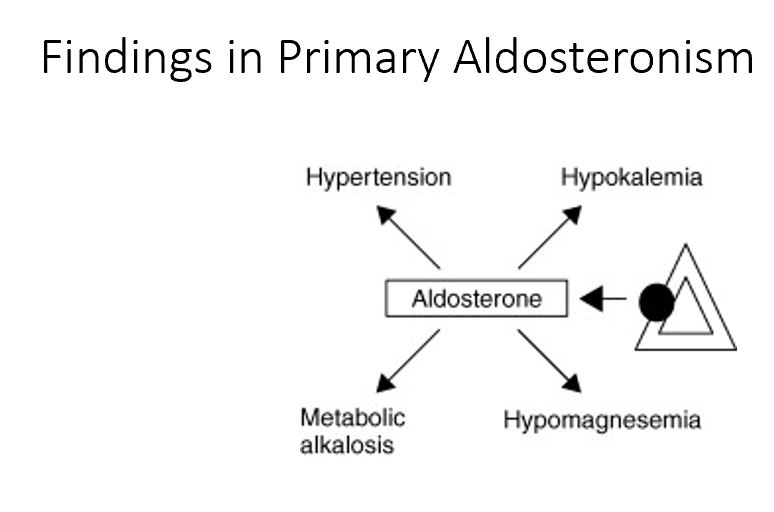
Main findings in primary aldosteronism
Hypertension
Hypokalemia
Metabolic alkalosis
Hypomagnesemia
What condition does Dawn have?
An appropriate screening test in this case is (initial step in documenting excess hormone):
primary aldosteronism
Ratio of aldosterone to plasma renin activity
(Plasma aldosterone has 90% sensitivity and specificity for the diagnosis of primary hyperaldosteronism)
An appropriate confirmatory test in this case is (second step-suppression):
Infusion of normal saline and assay aldosterone levels
(We need to test if aldosterone is working the way that it should.
Test this via increasing blood volume.
Increase blood volume -> inhibit renin -> inhibit aldosterone. If this doesn’t happen, aldosterone regulation isn’t working right.)

How would you expect Dawn’s acid-base status to change?
Lots of hydrogen ion which causes metabolic alkalosis
Conn’s Syndrome
AKA Primary Hyperaldosteronism
As this condition of hyperaldosteronism from a tumor continues on, there’s Na Escape Phenomenon (Na levels are returned to the high end of the normal range).
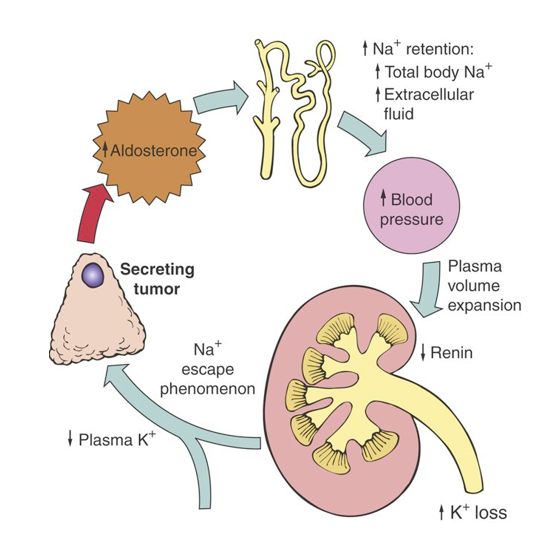
Addison’s Disease
AKA Primary Adrenal Insufficiency
Caused by:
Autoimmune
Metastatic disease
Adrenalectomy
Infectious adrenalitis
Adrenoleukodystrophy
Hemorrhagic infarction
Infiltrative
Drugs
Congenital adrenal hyperplasia

Debra’s clinical presentation, laboratory values, and lack of a response to the rapid ACTH test indicate her deficiency is in the:
Adrenal Gland


Absence of which hormone accounts for Debra’s relative hyponatremia, hyperkalemia?
Give an explanation for Debra’s hypotension. Why is Debra hypoglycemic?
ACTH
No, Cortisol means decreased BP (no stress) and more insulin present (less glucose).
(Primary adrenal deficiency = indicates issue in the adrenal gland = we lose aldosterone!)

The presentation of primary adrenal insufficiency is distinct from secondary adrenal insufficiency in that:
Primary adrenal insufficiency usually presents with hyperpigmentation
What hormones need to be replaced in primary adrenal insufficiency? Secondary adrenal insufficiency?
Primary – need cortisol and aldosterone
Secondary – don’t need to replace aldosterone but depending on what’s happening in pituitary, you may need to replace other hormones there. Depends on the true cause of this!
What hormone may be replaced but is not necessary in primary adrenal insufficiency?
Primary, adrenal androgens (especially if female). Adrenal gland is main source for androgens for females
How do doses of corticosteroids need to be adjusted during periods of stress in primary adrenal insufficiency? (for example, illness or surgery)
Increase it! You need more of it during stressful times because cortisol is the stress hormone.

What endocrine disorder should you consider in this patient?
Which other symptoms would you expect to see in Jennifer?
What test would you run first?
Hypercorisolism
Hypercortisolism: fatigue, weight gain, central adiposity, easy brusing.
24 hr urine free cortisol & Dexamethasone suppression test


What additional test would you like to order?
What do you suspect is the cause of Jennifer’s condition?
How will elevated androgen levels effect Jennifer?
Urine free 24 hour cortisol → Jennifer’s levels were 300 µg/dL levels (nl, < 90-100 µg/day)
Dexamethasone suppression test and 8 am cortisol sampling→ Jennifer’s levels were 25 µg/dL (nl, < 5 µg/dl)
ACTH → Jennifer’s levels were 120 pg/ml (nl, 6-76 pg/ml)
Cushing’s Disease
Menstrual irregularities and hirsutism (male pattern hair)


What medication was Jennifer likely prescribed?
What is the cause of her new symptoms?
What will the following lab values look like in Jennifer at this time?
Synthetic Glucocorticoid (to allow tissue to recover)
Adrenal Insufficiency
Cortisol = low, ACTH = low, Potassium = normal
(Cortisol will be low, ACTH will be low (due to lack of response of the normal tissue) and potassium will be normal (aldosterone levels will be normal in this case since aldosterone is not under primary control of ACTH))


What diagnosis should be suspected in John?
What is the basis for this diagnosis and why would this condition develop now?
What tests should be done to evaluate endocrine function?
Adrenal Insufficiency after an illness (because cortisol is low).
His dose of glucocorticoids was tapered to allow the corticotrophs to start producing ACTH. He was doing fine until a stressful event (illness) precipitated a need for additional cortisol which he was unable to response to adequately.
Cortrosyn (synthetic ACTH) stimulation test


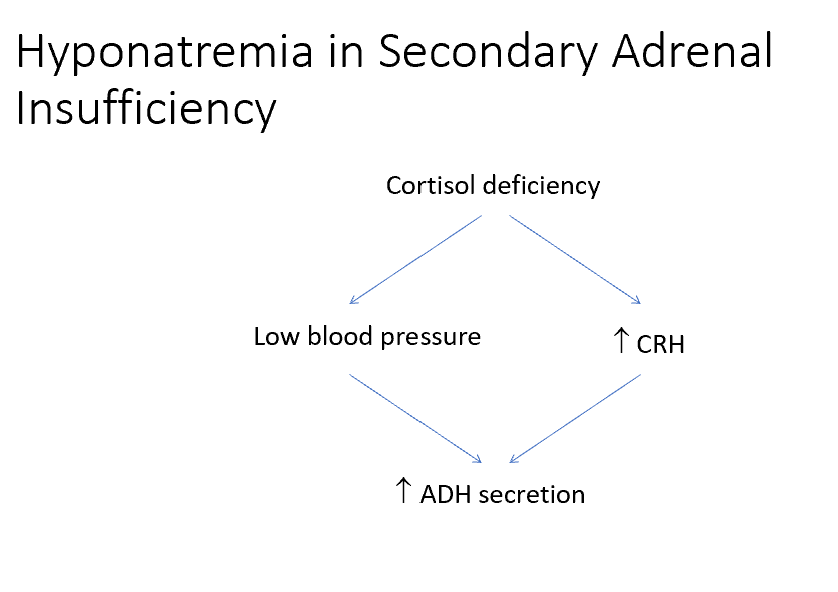
Explain the mechanism that could result in hyponatremia in this case of adrenal insufficiency.
Elevations in CRH can also trigger ADH secretion, leading to water retention and hyponatremia (see next slide for diagram)