Carbonyl Group Chemistry and Biochemistry
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
43 Terms
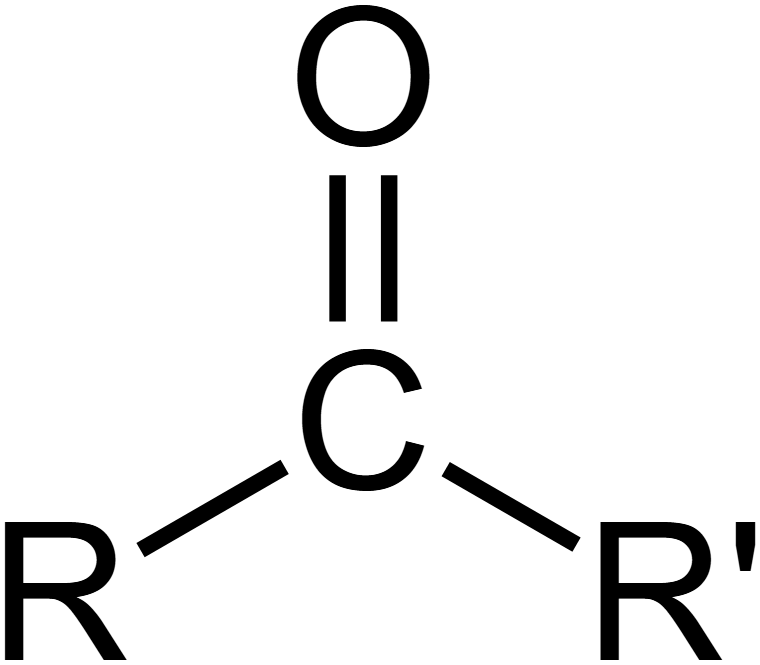
What is the carbonyl group?
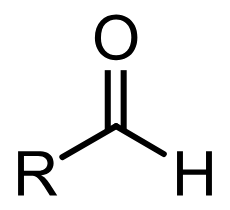
Aldehyde
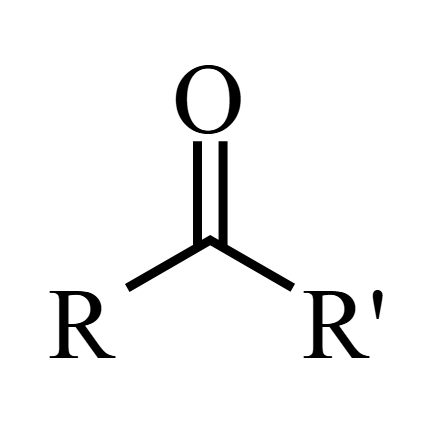
Ketone
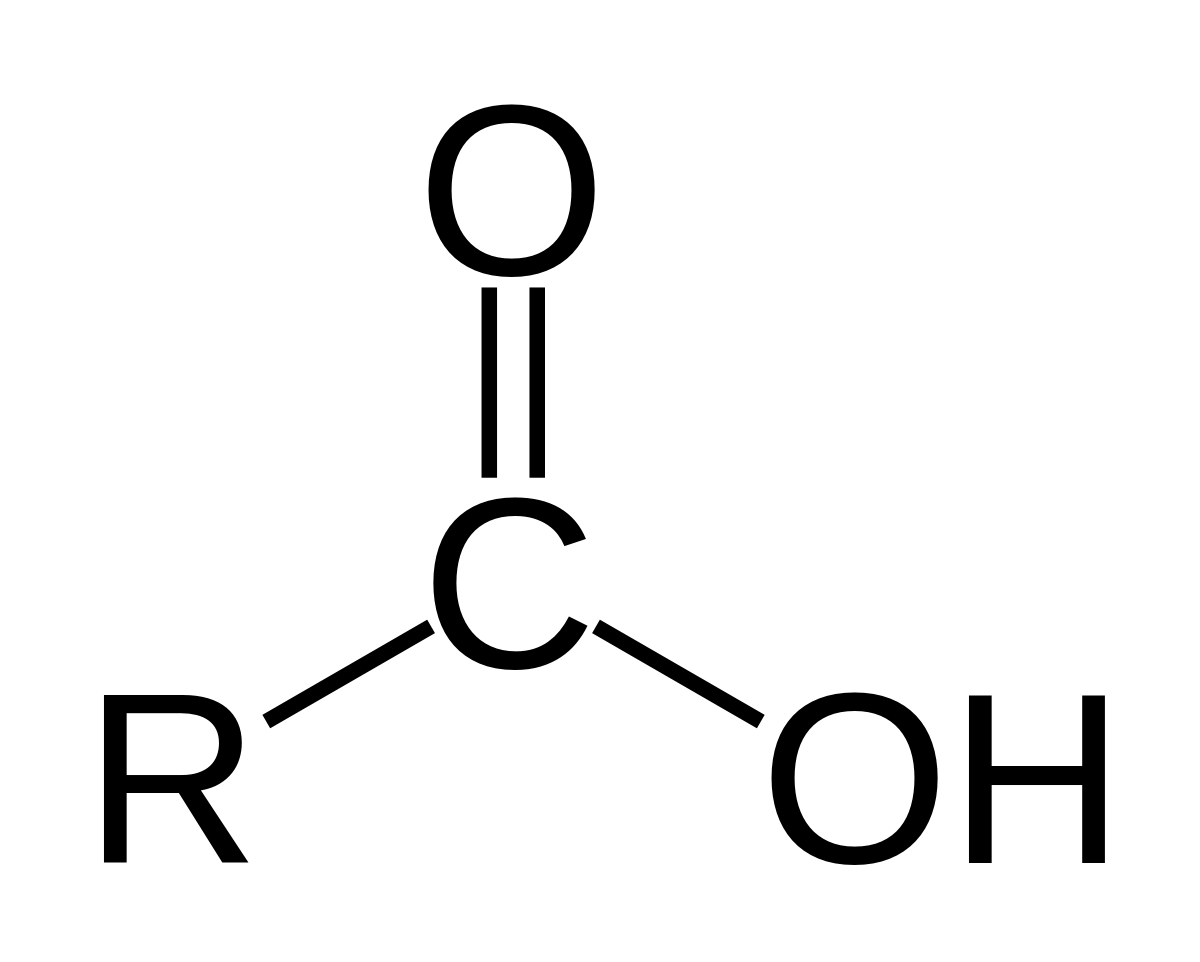
Carboxylic acid
Acyl chloride
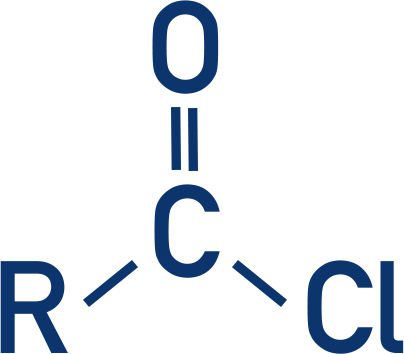
Ester
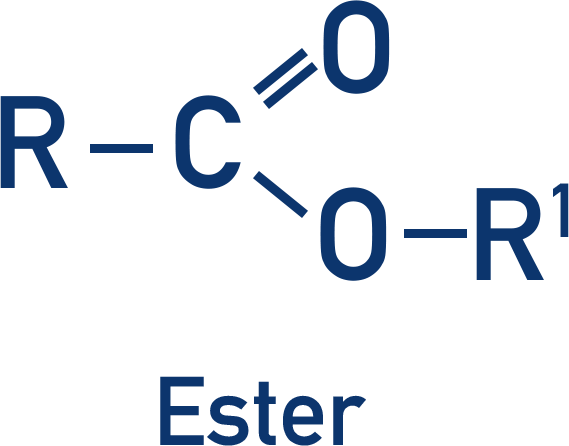
Thioester
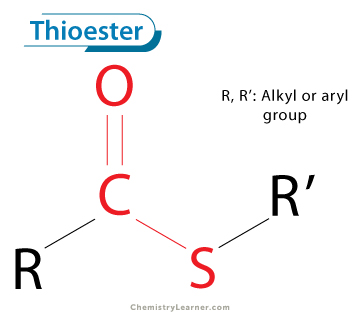
Amide
Acid anhydride
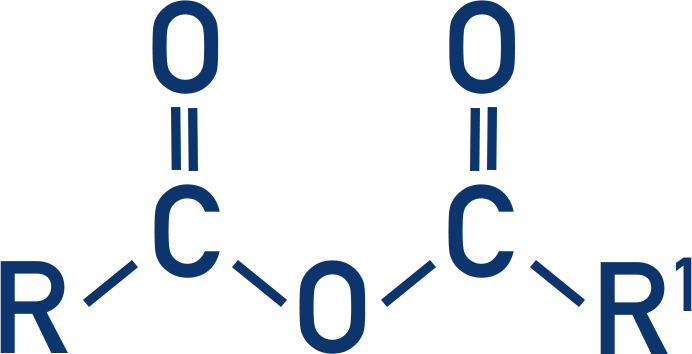
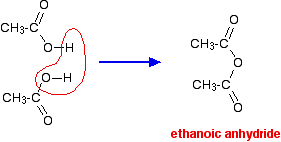
How are acid anhydrides formed?
Describe the reactivity of a carbonyl.
C=O bond is polar. O is δ⁻ (very electronegative), C is δ⁺.
Carbonyl carbon is electrophilic, so is susceptible to nucleophilic attack
The less electron density, the higher the δ⁺ charge on carbon, the more electrophilic the carbon is, the more reactive the carbonyl is in nucleophilic reactions.
Which bond is weaker, π and σ?
π bond
Which bond breaks first in the C=O double bond in a reaction?
The π bond is weaker so will always break first
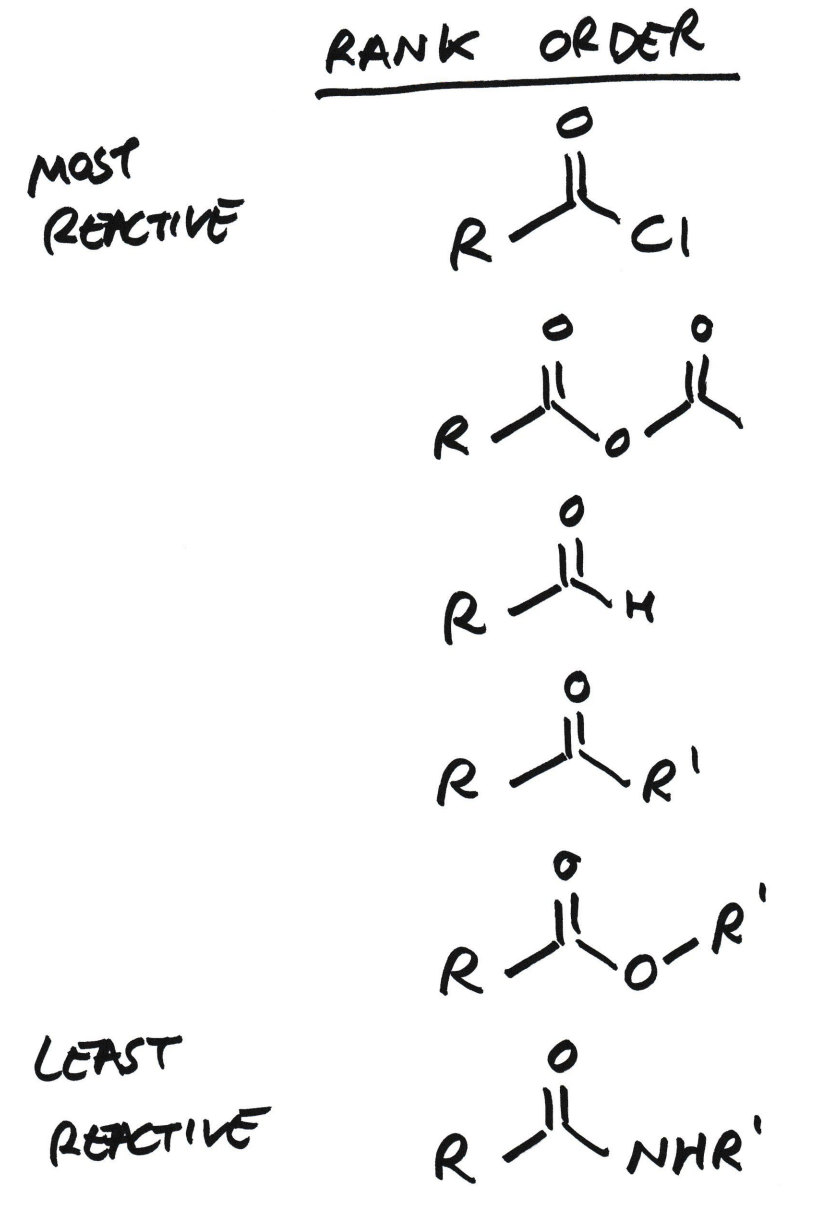
Order of reactivity for carbonyl bonds
What is the position of the C adjacent to the carbonyl?
α-position
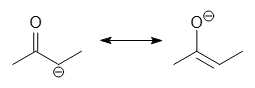
What are enolates?
Organic anions derived from the deprotonation of carbonyl compounds.
What is the pKa of HCl?
-6 to -7
Inductive effect leading to electron withdrawing:
This is -I (negative inductive effect)
What is -I / electron withdrawing?
A group pulls electron density toward itself through sigma bonds.
Common electron withdrawing groups (EWG) (-I groups):
HNCCCS
Have Never Cried Cooking Calamari Sauce
Halogens (-F, -Cl, -Br, -I)
-NO2
-CF3
-CN
-C=O (all carbonyl containing groups)
-SO3H
Are all carbonyl containing groups electron withdrawing (-I) or electron donating (+I)?
They are all electron withdrawing by induction (-I)
This is because in a carbonyl (C=O), oxygen is much more electronegative than carbon.
It pulls electron density toward the oxygen, making the carbonyl carbon partially positive.
This creates a strong -I (electron-withdrawing inductive) effect through the sigma bonds.
Inductive effect leading to electron donating:
This is +I (positive inductive effect)
What is +I / electron donating?
A group pushes electron density away from itself through sigma bonds.
Give some examples of common electron donating groups (EDG) (+I groups)
NOONO
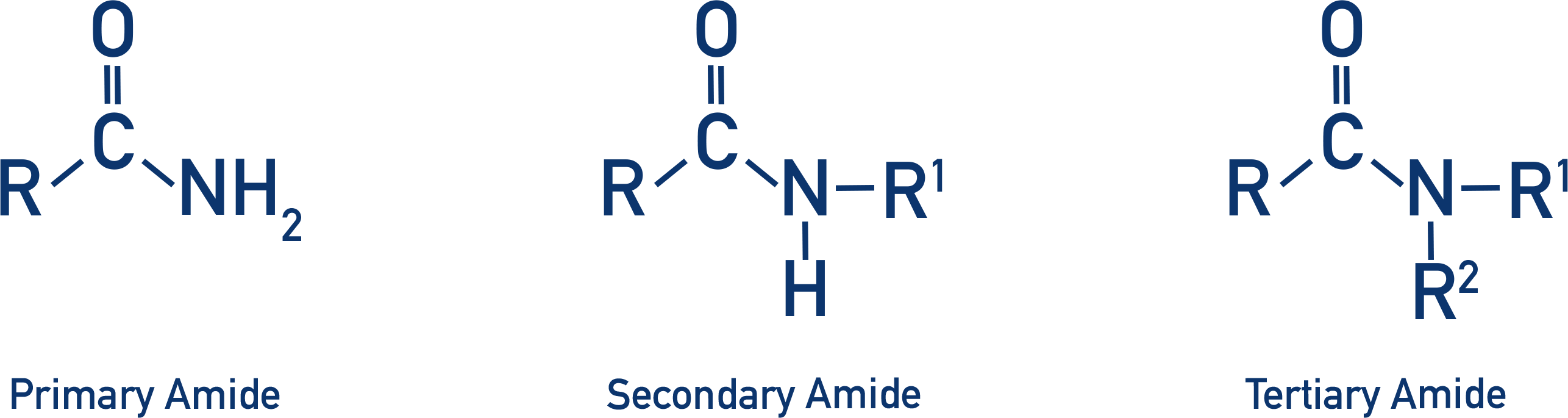
-NH₂, -NHR, -NR₂ (primary, secondary, tertiary amide)
-OH
-OR (alkoxy)
-NHCOCH₃ (amide on a benzene ring often donates into ring)
-O⁻ (phenoxide etc., extremely strong donor)
Electronegativity
N, O, F
(Cl is also electronegative)
Order the reactivity of carbonyls:
Acyl chloride
Acid anhydride
Aldehyde
Ketone
Carboxylic acid
Ester
Amide
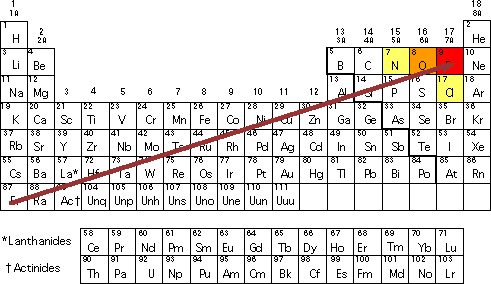
Why is acyl chloride the most reactive carbonyl group?
-Cl is a halogen and is highly electronegative, so it is an electron withdrawing group (-I group)
The inductive effect of Cl pulls the electron density out of the carbonyl C
Cl is pulling electrons away from something already δ+, making the C more electrophilic, so more reactive and susceptible to nucleophilic attack
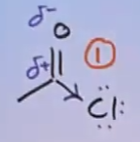
What is the mesomeric effect?
A lone pair of electrons adjacent to a double bonds
What’s the difference between the mesomeric effect and inductive effect?
Mesomeric effect occurs when a group can donate or withdraw electrons via resonance, not through sigma bonds (that’s inductive).
+M (electron donating by resonance)
Group donates electrons into a π system via lone pairs.
Example: -OH, -OR, -NH₂ attached to benzene.
Effect: increases electron density on the system → makes carbons less δ⁺ (less electrophilic).
-M (electron withdrawing by resonance)
Group pulls electrons away from a π system into itself.
Example: -NO₂, -C=O, -CN.
Effect: decreases electron density on the system → makes carbons more δ⁺ (more electrophilic).
Quick way to remember +M and -M
+M
Lone pair → π system
Increases electron density, so less δ⁺
-M
π electrons → group
Decreases electron density, so more δ⁺
Why doesn’t the mesomeric effect affect the reactivity of acyl chloride, considering that Cl has 3 lone pairs of electrons and is adjacent to a double bond (+M), it should increase the electron density, surely?
Chlorine is one below carbon in the periodic table
This means chlorine is much bigger than carbon
Therefore the mesomeric effect is very poor
Why is aldehyde more reactive than the ketone?
Alkyl groups are weakly electron donating. They are electron donating groups (EDG, +I groups), so is donating electrons to the δ+ C, making the C less electrophilic, so less reactive and less susceptible to nucleophilic attack
In ketone, there are 2 alkyl groups. In aldehyde, there is 1 alkyl group and 1 H
Therefore ketones experience 2x inductive effect (+I) compared to aldehyde

Why is carboxylic acid reactivity only moderate?
Carbonyl carbon is electrophilic (δ⁺) due to the C=O bond.
The oxygen is -OH is very electronegative, so is a strong electron withdrawing group, so is carbonyl C becomes more electrophilic (makes δ+ more reactive) and more susceptible to nucleophilic attack
However, the carboxylic acid still isn’t very reactive
This is because oxygen is in the same row as carbon in the periodic table. They are very similar size. The mesomeric effect is occurring because oxygen has lone pair adjacent to the double bond. The lone pair is being donated to the π bond, increasing electronegativity (less δ+)
The overlap of mesomeric effect and inductive effect is very effective. Pulling electrons is inductive, pushing electrons is mesomeric. Taking both effects into account, it isn’t very reactive
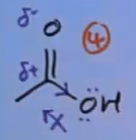
Why is amide the least reactive?
The nitrogen has a lone pair, so is +M and is increasing electron density, so the carbonyl C becomes less δ⁺
It is also electron withdrawing via the inductive effect, but not as electron withdrawing as oxygen because N is less electronegative than O (N,O,F)
What is the acetyl group?
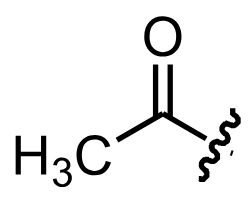
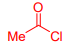
What is Acetyl chloride?
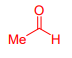
What is Acetaldehyde?
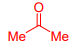
What is Acetone?
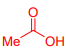
What is Acetic acid?
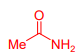
What is Acetamide?
Are amides basic?
No
Basicity depends on how available a lone pair is to accept H⁺.
Amides are not basic because the nitrogen lone pair is delocalised into the carbonyl by resonance and cannot readily bind H⁺.