Parasitolgy Exam 3
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
98 Terms
Parasitic protozoan characteristics
-unicellular
-widely dispersed
-heterotrophic
Parasitic Protozoan characteristics (continued...)
-immune evasion (change antigen make up)
-Multiplying in host (sudden onset symptoms)
-DEPENDENT ON host
-Simple or complex
-Asexual vs sexual life cycles (makes it very complex dependent on organism)
Parasitic protozoan classes
Amoeba – endemic in US
·Ciliates – endemic in US
·Flagellates – some endemic / some exotic
·Sporozoites – all exotic
Parasitic protozoan groups
intestinal and blood and tissue (systemic infection)
Parasitic protozoan: Types of locomotion
Amoeboid movement
Flagella
Cilia
Non-motile
General life cycles (Protozoans)
Direct transmission-sexual intercourse and kissing. NEVER leaves the host and changes within host Ex. trichomoniasis
General life cycles (Protozoans)
Fecal/ Oral transmission: USUAL through environmentally resistant cyst via fecal or oral. likely gets into water and food. Ex. Entamoeba histolytica
General life cycle (Protozoan)
Vector borne transmission: Likely to be through arthropods during blood meals. Vectors are usually intermediate/definitive host Ex. Trypanosoma brucei
General life cycle (Protozoan)
Parasites in prey TISSUES taken up through the predators (ecological circle) ex. Toxoplasma gondii
Trophozoite form (PP life cycles)
multiplication by binary fission - nuclear division and cytoplasmic division (usually the growth and reproduction)
Cyst form
- Protective form of a protozoan that enables it to survive in harsh environments
- Allow survival outside the host and passage from one host to another
Cyst environmental triggers
Excess food
Reduced food
Excess Metabolic waste products
Desiccation
oxygen level (too much oxygen)
Temperature
Cyst types
Protective - Cyst wall protects organism - no morphological changes (Balantidium)
Reproductive - Cyst protects parasite, nuclei divide, increase in number of trophozoites exiting the cyst (entamoeba histolytica)
Entamoeba histolytica (Amebiasis): Morphology
Trophozoite amoeboid form in mucosa and tissues
RBCs in cytoplasm
10-20um
Explosive pseudopodia
Growth and feeding stage
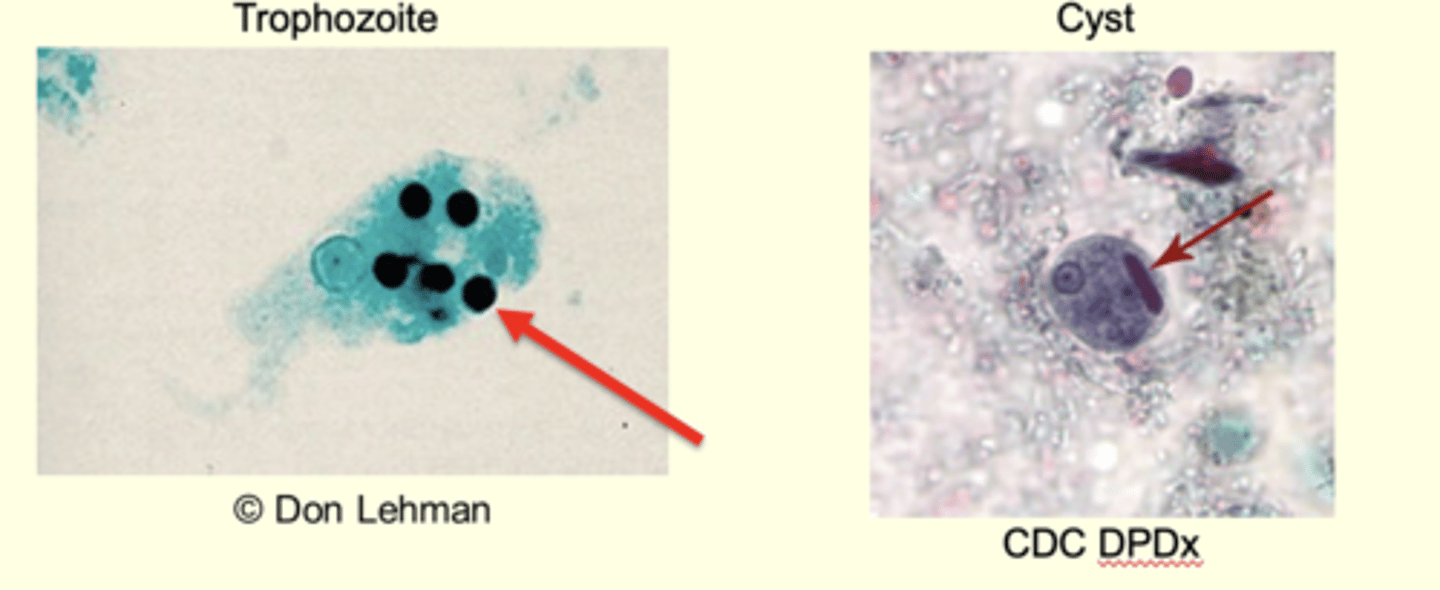
Entamoeba histolytica: Morphology Cyst
Reproductive cyst
Resistant stage
1-4 nuclei
10-20 microns in diameter
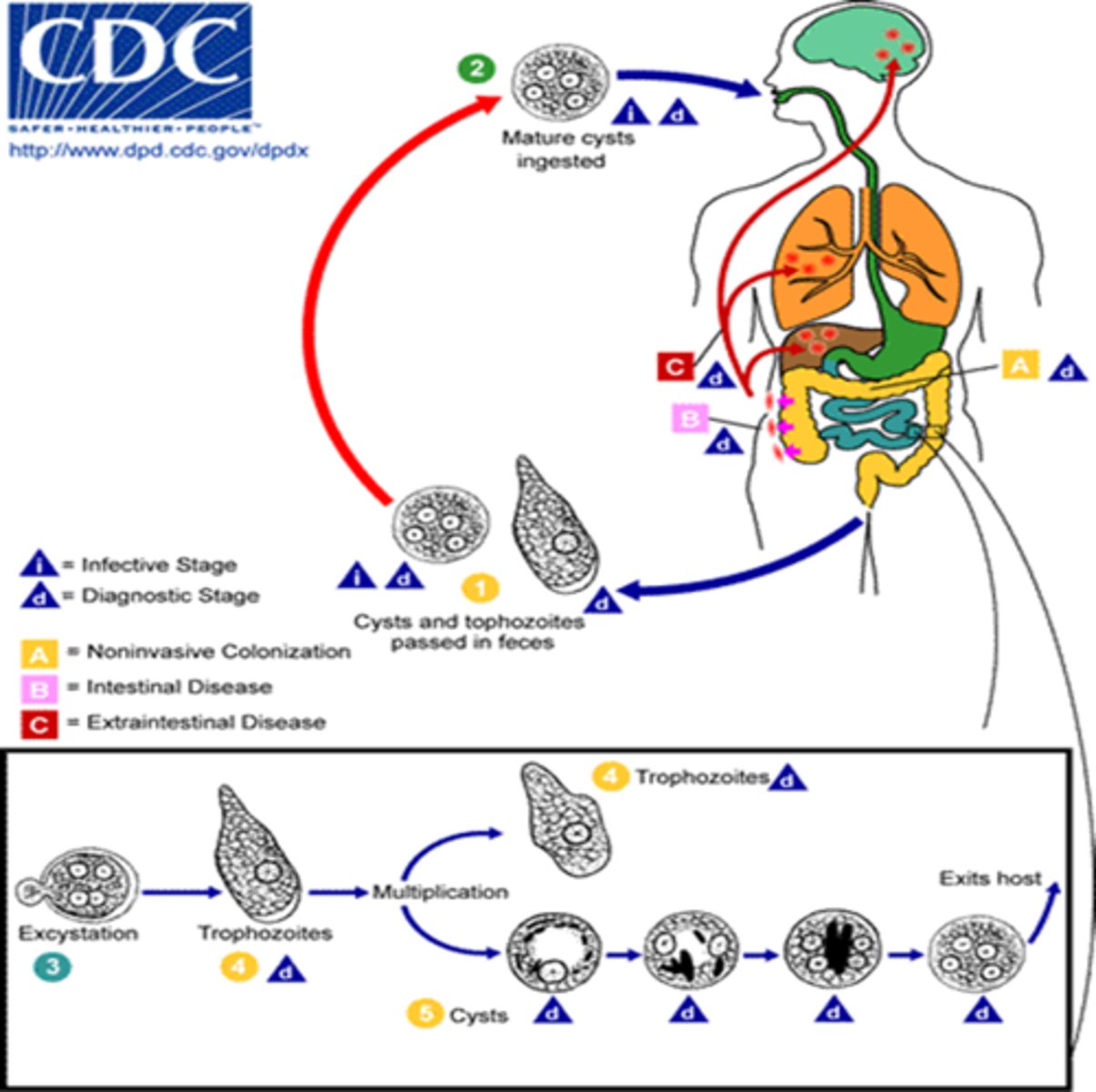
Entamoeba histolytica life cycle
1) Ingestion (infective stage): Humans ingest mature cysts in contaminated water/food or hands
2) Excystation: In the small intestine the cyst wall breaks (excystation) releasing small trophozoites that then migrate to the large intestine.
3) Trophozoites multiply and can invade (diagnostic stage also): In the colon, trophozoites multiply by binary fission and feed on bacteria/mucus.
Some remain on the mucosal surface.
Others invade the intestinal wall, creating flask-shaped ulcers, and may enter the bloodstream to reach the liver (most common), lungs, or brain.
4) Encystation and shedding (diagnostic stage): In the colon, some trophozoites encyst, forming new mature cysts that are excreted in feces.
Entamoeba histolytica epidemiology
-50 million cases
-100K deaths
-10 to 20% Symptomatic
-80 to 90% Asymptomatic
-2nd leading cause to parasitic diseases
Intestinal and extra-intestinal Entamoeba histolytica
Intestines (large intestines)
Extra-intestinal
·Liver- via mesenteric blood vessels or erosion through gut
·Lungs - via gut erosion
·Skin - via gut erosion and erosion of abdominal wall
·Brain - via circulation usually from a liver abscess
Entamoeba histolytica pathology Acute intestinal stage
1st stage: tear drop or flask shaped ulcer
2nd stage: Break through the submucosa spread laterally
Most pathology on SURFACE
Entamoeba histolytica pathology
Chronic intestinal stage symptoms
Lower back pain
Fatigue
Pain in lower right quadrant
Entamoeba histolytica diagnosis (Extra intestinal and intestinal)
-Looking through feces (intestines)
-Coughing and aspirating (extra intestinal)
Entamoeba gingivalis
associated with infected gums:
-Mouthwashing and brushing
-Trophozoites in mouth
-good oral hygiene
Acanthamoeba sp life cycle
Cysts (Stage 1)
The cyst is the infective form of Acanthamoeba.
Found in soil, water, and air, these cysts can survive harsh conditions.
Trophozoite (Stage 2)
When conditions are favorable, the cyst transforms into a trophozoite, the active feeding and dividing form.
Both cysts and trophozoites can infect humans.
Mitosis (Stage 3)
Trophozoites reproduce by simple mitotic division (asexual reproduction).
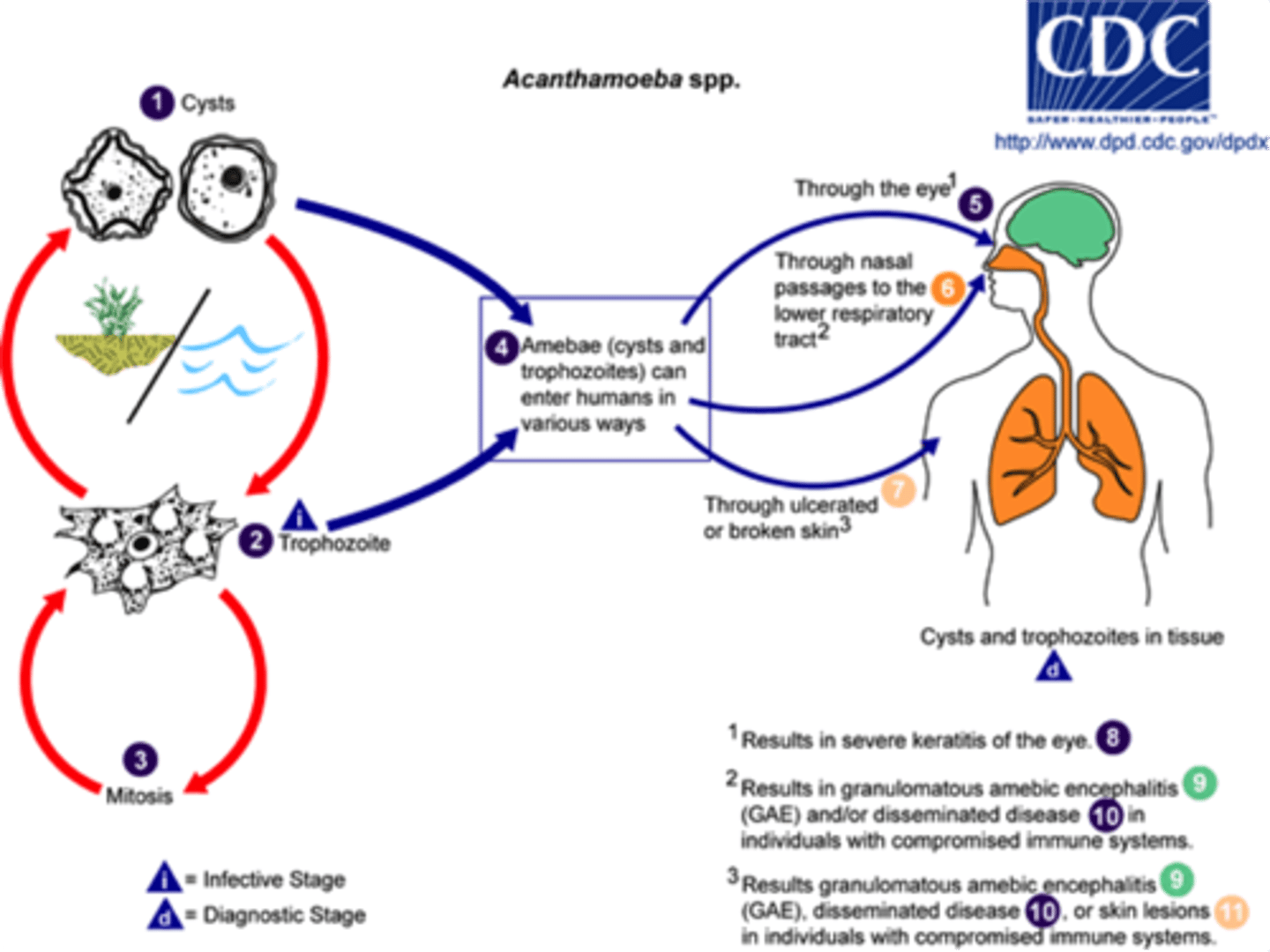
Acanthamoeba spp. epidemiology and pathology
Free-living and freshwater living usually in sewage, swimming pools, and tap water
Keratitis and granulamatous amoebic encephalitis
Granulomatous amoebic encephalitis
Acanthamoeba species: Infection CNS death of 95% enter through respiratory tract and travels to brain thru blood
Keratitis
Corneal ulceration (contact lens solution ineffective at prevention of acant spp)
Naegleria fowleri Life cycle
1.) cyst stage in water cycles between trophozoite and cyst stage
2.) trophozoite alternates with a flagellated form
3.) transition to amoeboid stage to infect humans
4.) enters brain through olfactory nerves then causes primary amebic meningoencephalitis (PAM)
5.) you dead
-only ameboid stage in humans
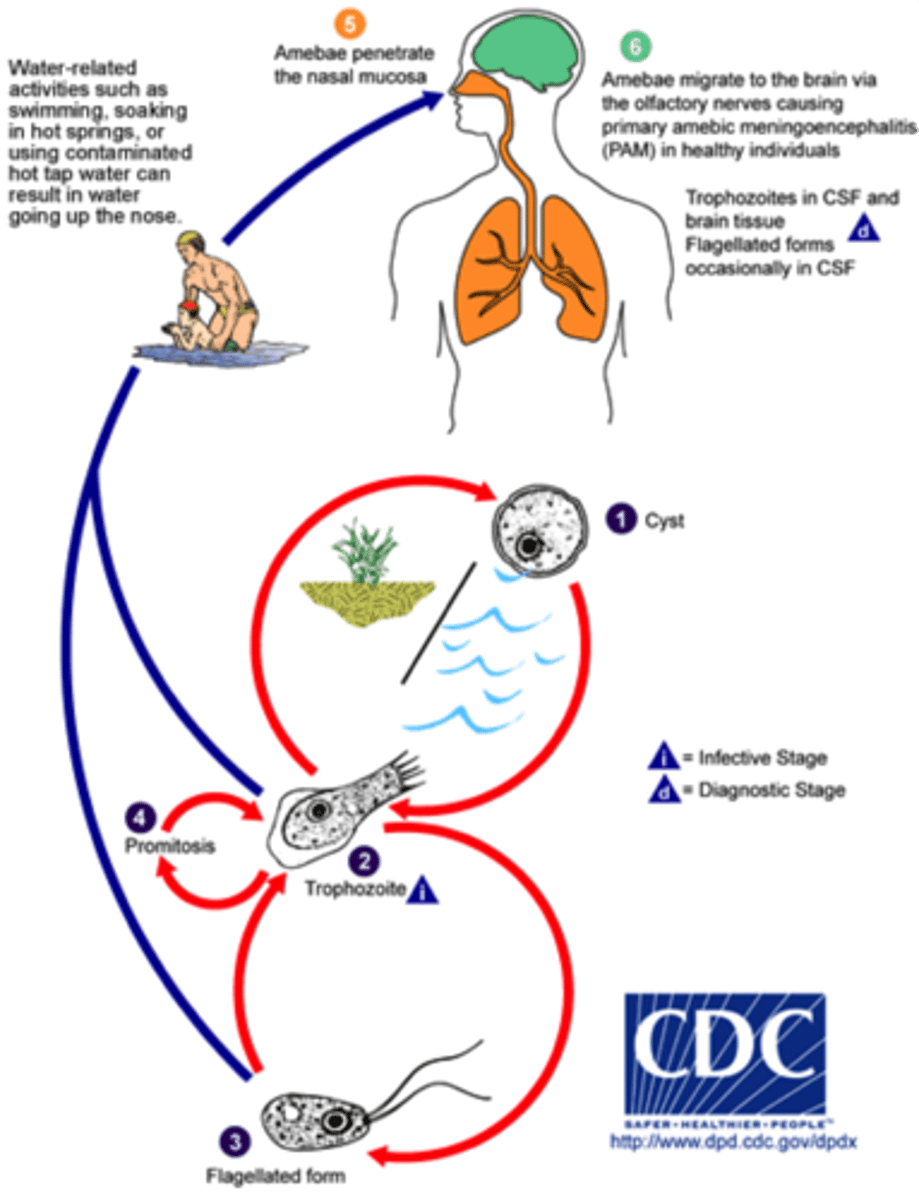
Primary Amoebic Meningoencephalitis
Severe brain infection caused by Naegleria fowleri. inhaled through nose enters through the nasal cavity and olfactory nerve tissue. Death is imminent
Naegleria fowleri epidemiology
Found in warm freshwater such as ponds and lakes. Heat resistant protozoan. Found in poorly chlorinated waters
Balantidium coli life cycle
Cyst stage: People swallow cysts from food or water contaminated with pig feces.
Excystation: The cyst opens in the small intestine, releasing a trophozoite.
Trophozoite stage: The trophozoite lives in the large intestine, feeding and multiplying.
Invasion: Some trophozoites burrow into the intestine wall, causing ulcers and diarrhea.
Encystation: Trophozoites turn back into cysts before leaving the body.
Cyst release: Cysts exit in feces and can infect new hosts, restarting the cycle.
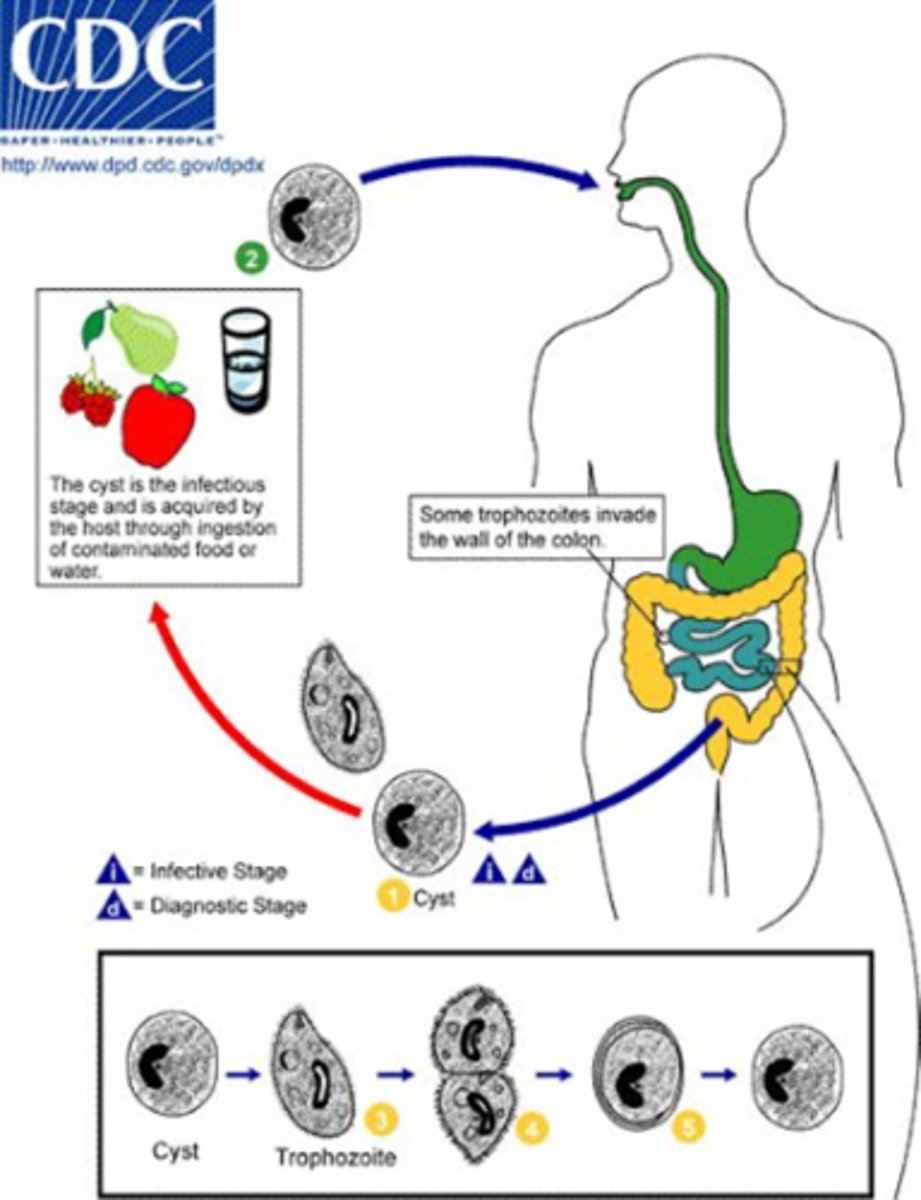
Balantidium coli epidemiology
Not a common human pathogen
Seen more commonly in tropical regions
Associated with contact with pigs (reservoir)
Balantidium coli diagnosis
Cyst or cilate stage in POOP
balantidium coli pathology
humans are natural resistors
burrows itself into mucosa
tissue invader
What are the main types of parasitic protozoans?
Atrial and intestinal flagellates, blood and tissue flagellates.
Name three examples of parasitic flagellates.
Leishmania, Giardia, Trichomonas, and Trypanosoma.
What is Trichomonas vaginalis commonly associated with?
It is a common sexually transmitted disease (STD).
What is the prevalence of Trichomonas vaginalis in females compared to males?
More common in females (2.1%) and less common in males (0.5%).
What are the common symptoms of Trichomonas infection in females?
Vaginitis, puritis, burning, vaginal discharge, and irritation.
What treatment is recommended for Trichomonas infections?
Metronidazole or Tinidazole, both at 2 g orally in a single dose.
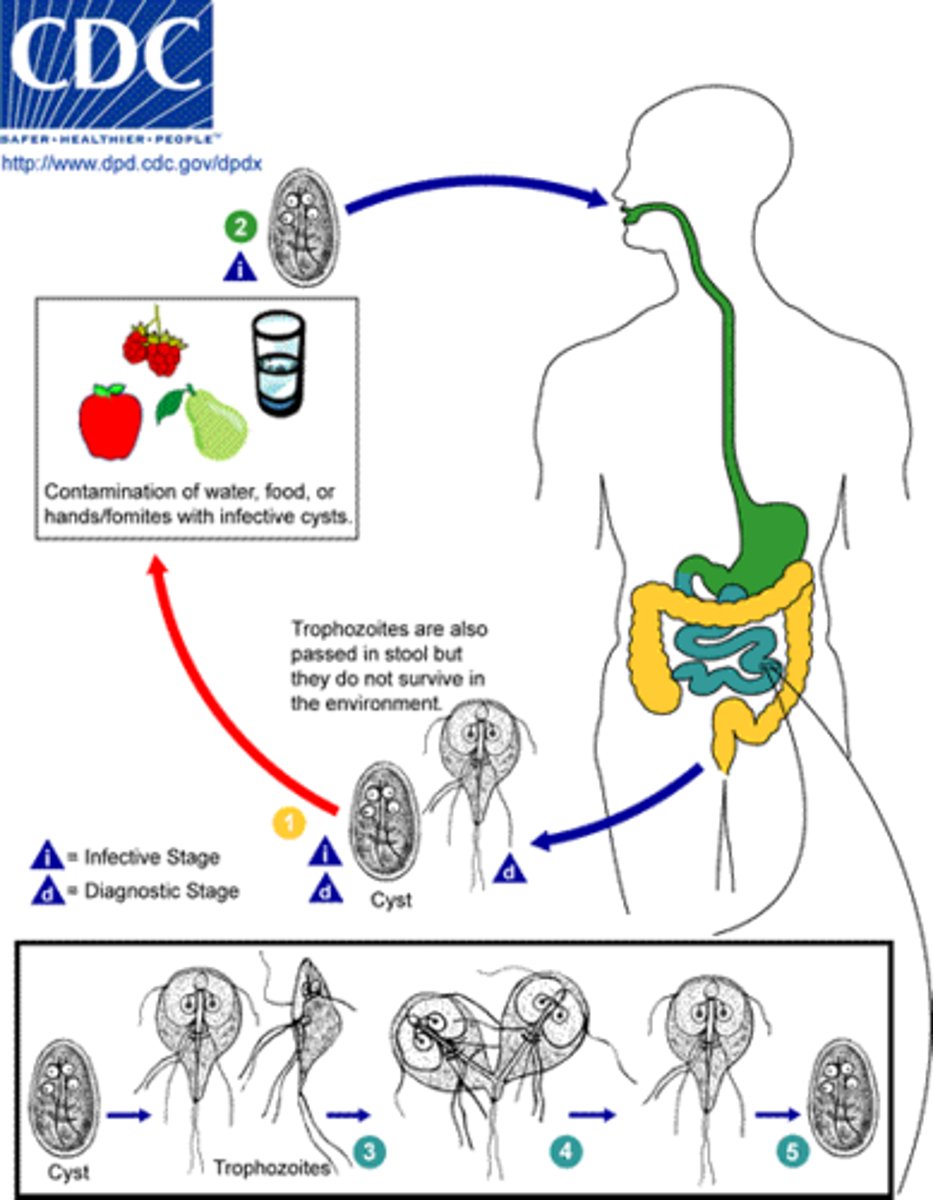
What is Giardia lamblia's primary mode of transmission?
Transmission occurs through ingestion of cysts, often via fecal contamination.
What are the common symptoms of Giardia lamblia infection?
Malabsorption, inflammation, and diarrhea.
How can Giardia lamblia be diagnosed?
By identifying cysts in stool or liquid stools and considering exposure history.
What is the treatment for Giardia lamblia infections?
Metronidazole at 250 mg for 5 to 7 days.
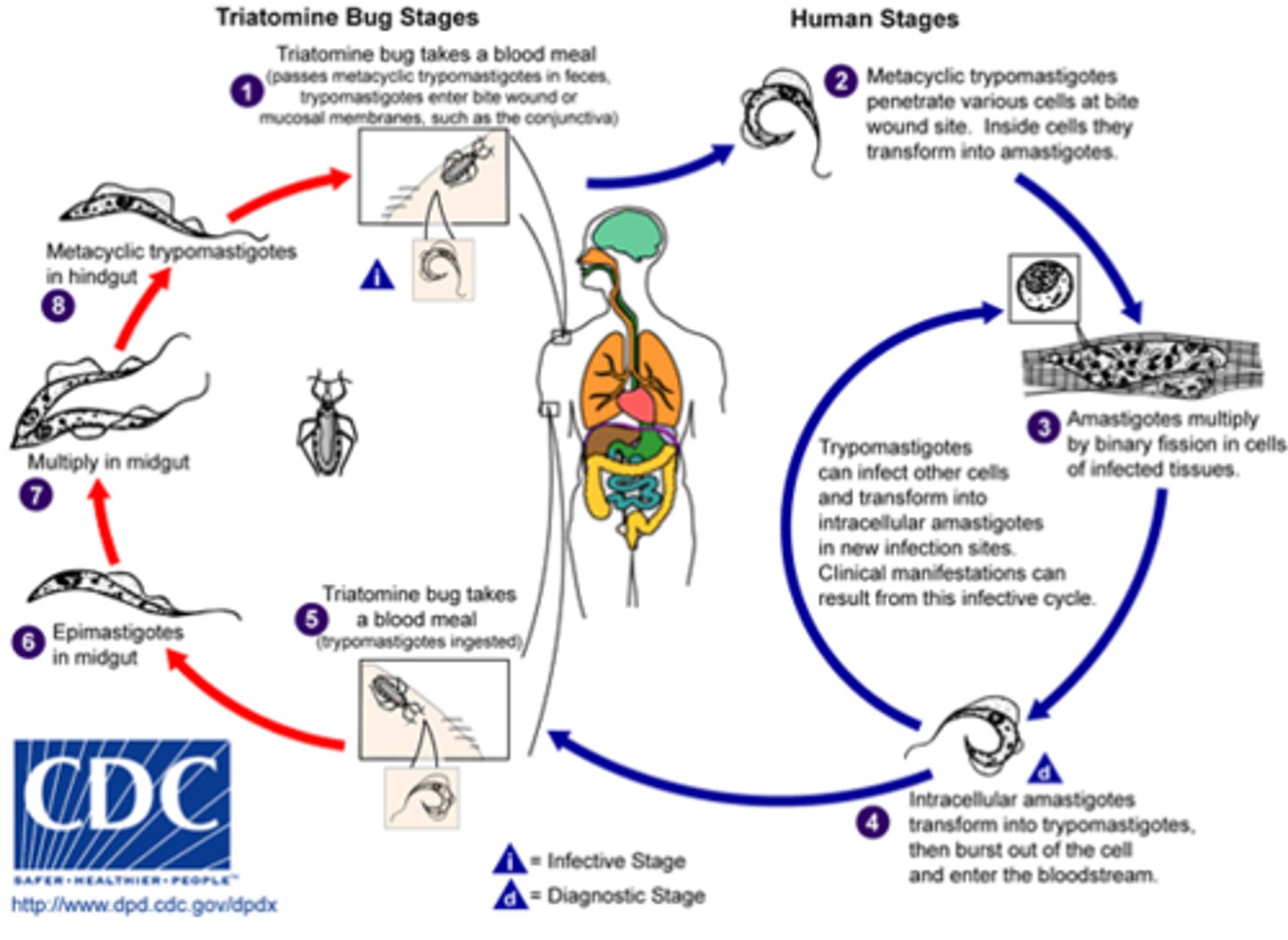
What is Chagas Disease caused by?
Trypanosoma cruzi.
What is the primary vector for Trypanosoma cruzi?
Reduvidae bugs, commonly known as 'kissing bugs.'
What are the three phases of Trypanosoma cruzi infection?
Acute phase, intermediate phase, and chronic phase.
What are common symptoms during the acute phase of Chagas Disease?
Fever, hepatomegaly, splenomegaly, and Chagoma.
What is the chronic phase of Chagas Disease characterized by?
Megaesophagus, megacolon, and cardiomegaly.
What is the treatment success rate for Chagas Disease in infants?
90% cure rate.
What is African trypanosomiasis caused by?
Trypanosoma brucei gambiense and Trypanosoma brucei rhodiense.
What is the vector for African sleeping sickness?
The Tsetse fly.
What is a key symptom of the acute stage of African sleeping sickness?
Winterbottom's sign, which is lymph node enlargement.
What are the symptoms of the chronic stage of African sleeping sickness?
CNS involvement leading to confusion, lassitude, inactivity, coma, and death.
What is the treatment for early-stage African sleeping sickness?
Suramin for hemolymph stage before CNS involvement.
What preventive measures can be taken against African sleeping sickness?
Kill the vector, use insecticide-impregnated clothing, and apply repellents.
What is the estimated global risk for African sleeping sickness?
Approximately 60 million people in 36 sub-Saharan African countries.
What is the prevalence of Giardia lamblia in developed vs. developing countries?
2-7% in developed countries and 20-30% in developing countries.
What are the common diagnostic methods for Chagas Disease?
Blood sample for trypomastigote forms, serology, and electrocardiogram.
Giardia lamblia
Ingestion of cysts (contaminated food or water)
↓
Excystation in duodenum → trophozoites released
↓
Trophozoites attach to intestinal mucosa and multiply
↓
Encystation in colon → cysts formed
↓
Cysts excreted in feces → contaminate water/food
↓
Ingestion by new host → cycle repeats
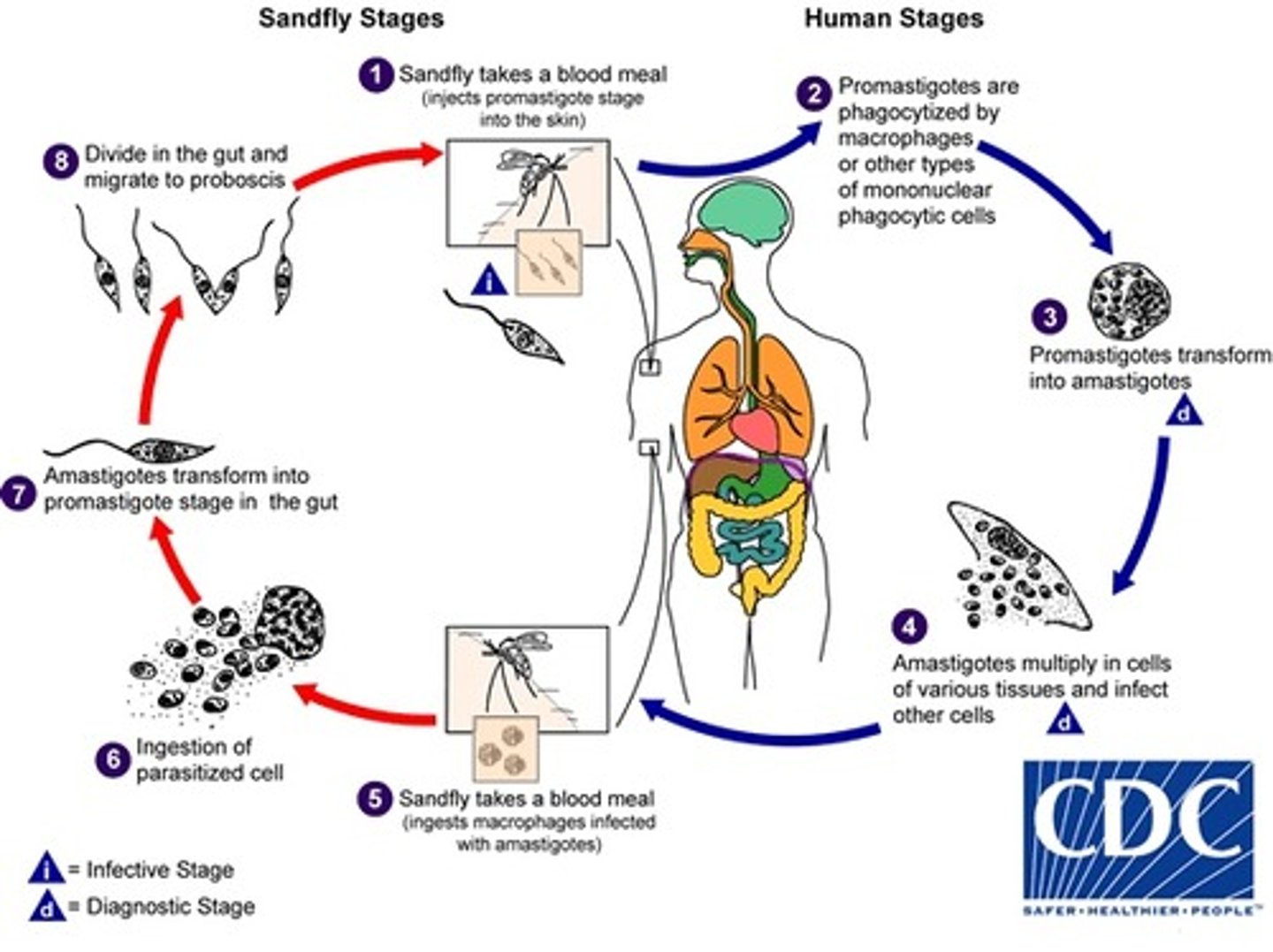
Leishmania donovani/tropica
1. Sandfly (phlebotomous papatassi) takes blood meal (injects promastigotes stage )
2. Promastigotes are phagocytized
3. Promastigotes transforms in to amastigotes
4. Amastigote multiply
5. Sandfly takes blood meal
6. Ingestion of parasitized cell (with amastigotes)
7. Amastigotes transform into promastigotes in midgut ofsandfly
8. Divide in midgut and migrate to proboscis
9. Bite an- other person
INTERMEDIATE HOST: s
Trichomonas vaginalis
Trophozoite (in host)
↓ (asexual binary fission)
More trophozoites
↓ (sexual contact)
Transmission to new host
↓
Colonization of urogenital tract
↓
Replication → Continued infection
Trypanosoma brucei
Tsetse fly bites → injects parasite → parasite spreads and multiplies in human → fly bites infected person → parasite grows inside fly → fly bites new person → infection continues.

Trypanosoma cruzi
Life cycle there
What are leishmanial parasites?
A group of hemoflagellate parasites that cause leishmaniasis.
What are the four complexes of Leishmania
Leishmania tropica (cutaneous)
Leishmania mexicana (cutaneous)
Leishmania donovani (visceral)
subgenus Viannia (Mucocutaneous)
What is the primary vector for Zoonosis?
Phlebotomous sp. (sand fly). Primarily affects dogs
Zoonosis
an infection indigenous to animals but naturally transmissible to humans
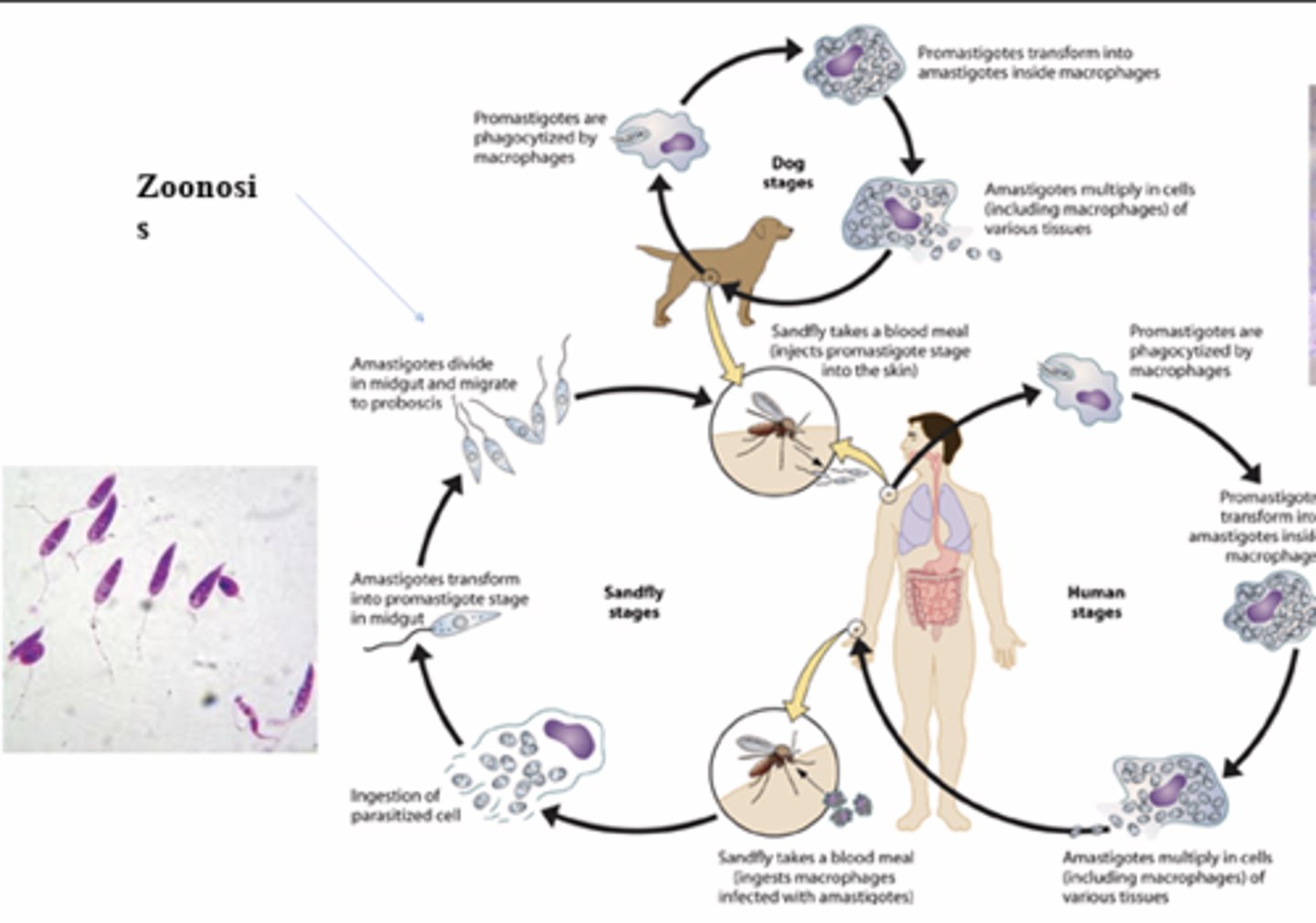
What the of life cycle mastigotes
Promasigote to amastigote to promastigote
What is the global impact of leishmaniasis in terms of infection rates?
More than 12 million infected people, with 0.9 to 1.6 million new cases each year.
What are the common symptoms of visceral leishmaniasis?
Fever, anorexia, weight loss, diarrhea, hepatomegaly, splenomegaly, anemia, and tissue and muscle wasting. Ranging from 10 days to 2 years dependent on severity
What type of examination is used for diagnosing leishmaniasis?
Microscopic examination of tissues for amastigotes.
What types of tissues are examined for amastigotes in leishmaniasis diagnosis?
Skin, mucous membranes, and internal organs.
What are the two forms of leishmaniasis based on geographical distribution?
Old World Leishmaniasis and New World Leishmaniasis.
What is cutaneous leishmaniasis associated with?
Skin lesions, which can be single or multiple.
What areas are affected by mucocutaneous leishmaniasis?
Mucous membranes, including the nose, oral cavity, and genital areas.
Leishmaniasis Control
Insecticide, Vaccinations, eliminating malnutrition, reservoir control
What is the genus that describes organisms that infect humans, monkeys, and apes?
Plasmodium sp.
Which five species of Plasmodium regularly infect humans?
Plasmodium vivax, Plasmodium malariae, Plasmodium falciparum, Plasmodium ovale, Plasmodium knowlesi.
What is the primary difference in liver phase between P. falciparum and the other species?
P. falciparum has no chronic liver phase, while P. vivax, P. malariae, P. ovale, and P. knowlesi have a persistent liver phase.
What is the definitive host for the malaria parasite?
Anopheles mosquito.
What is the incubation period for malaria?
9 to 40 days from the bite to the onset of signs and symptoms.
What are the common signs and symptoms of malaria?
Headache, lassitude, fever, and chills.
What is a malarial paroxysm?
Regular intervals of fever and chills associated with malaria.
What fever cycle is associated with P. vivax and P. ovale?
Benign tertian - 3 day cycle.
What fever cycle is associated with P. malariae?
Quartan - 4 day cycle.
What fever cycle is associated with P. falciparum?
Malignant tertian - 3 day cycle.
What is acute benign malaria and which species are involved?
Acute benign malaria is caused by P. vivax, P. malariae, and P. ovale, characterized by headache, malaise, chills, and fever.
What are the characteristics of acute malignant malaria?
Caused by P. falciparum, it can lead to cerebral malaria, increased concentration of parasitized RBCs in brain capillaries, and can result in coma or death.
What is Blackwater fever and which species causes it?
A severe condition caused by P. falciparum, characterized by renal failure and hemolytic syndrome.
What is malarial cachexia?
A generalized state of debility marked by anemia, jaundice, splenomegaly, and emaciation due to chronic malarial infection.
What are the general pathologic changes in blood due to malaria?
Anemia, multiple thrombosis, and reduced blood volume.
What causes hepatomegaly in malaria?
Phagocytosis of merozoites and infected RBCs by Kupffer cells.
What is the gold standard for diagnosing malaria?
Thin and thick blood smears, along with observation of signs and symptoms.
What are some prevention methods for malaria?
Treating carriers, mosquito control, screens and netting, and prophylactic drugs.
How long does immunity last for P. falciparum and P. vivax?
Immunity lasts about 1 year for P. falciparum and about 1.5 years for P. vivax.
What is the estimated number of new malaria cases in 2016?
216 million new cases.
How many deaths were attributed to malaria in 2017?
650,000 deaths, with 90% occurring in Africa.
What is the role of Anopheles mosquitoes in malaria transmission?
Only female Anopheles mosquitoes transmit malaria.