MICR2000 - Environmental Microbiology
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
Pure culture methods of measuring microbial diversity
culture dependent
e.g., streaking
good for growing things that use sugars
difficult to replicate environmental conditions in lab
unknown pH, temp, salinity, partner requirements (microbial food webs
can’t culture all types of microbes - not good representation of microbial diversity
Great Plate Count Anomaly
viable plate count and most-probable-number techniques underestimate true diversity of microorganisms (<1%) can be cultured in lab
Culture independent method of measuring microbial diversity
using DNA or RNA - typically 16S rRNA
phylogeny
evolutionary history of microorganisms using gene sequences
objective measurement of diversity
What is needed for a DNA marker gene for phylogeny?
large macromolecules (high information content)
universally distributed since start of evolution
functionally constant
slowly changing in sequence
relative change constant across all microorganisms so we can compare
not too slowly that we miss differences bc of reversions
examples
ATP synthases (ATPases)
DNA polymerases
Ribosomal rRNAs
Importance of 16S rRNA gene
Meets requirements for evolutionary chronometer (good marker gene)
e.g., not too many or too little bases
Part of SSU (small subunit) of prokaryote ribosomes
most phyla of bacteria and archaea are represented only by environmental 16SrRNA sequences as they couldn’t be cultivated
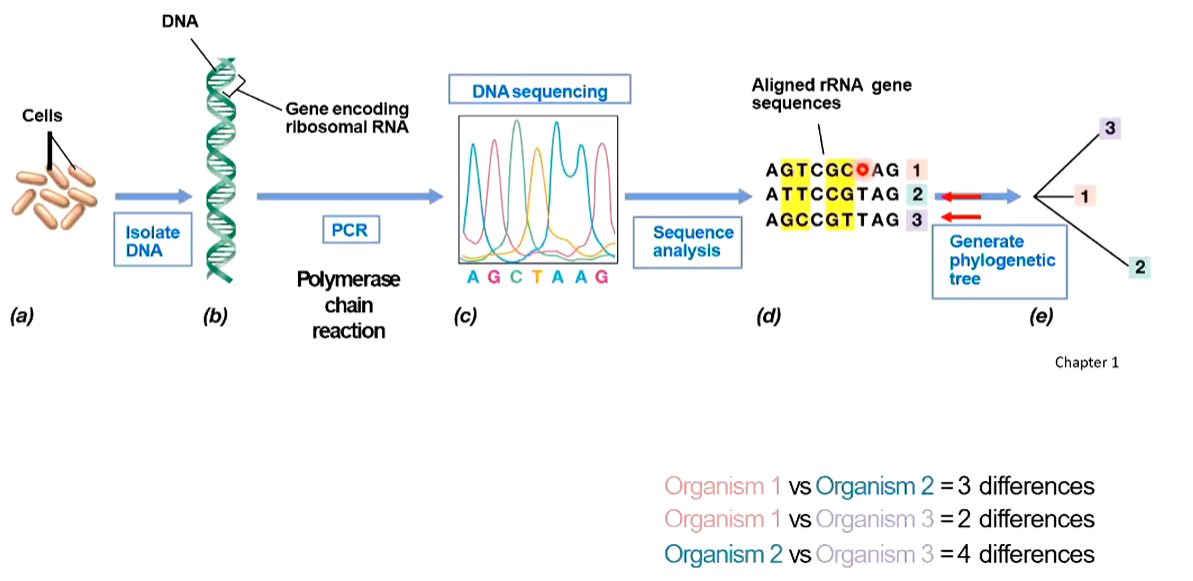
Gene sequencing steps/how to make a phylogenetic tree
Isolate DNA (of separate cell types to compare)
PCR amplification
Sequence PCR amplified genes (AGTC sequence)
Sequence analysis
align rRNA gene sequences
compare differences
Generate phylogenetic tree
based on relatedness
length of branches indicates number of differences
3 lineages of cells
3 domains
Bacteria (prokaryotic)
Archaea (prokaryotic)
Eukarya (eukaryotic)
Which bacteria on the phylogenetic tree are closer to LUCA?
thermophiles - like hot environments, suggesting LUCA originated in thermal environments
Major Bacterial Phyla
Proteobacteria
medically relevant such as e. coli, klebsiella
gram +ve bacteria
cyanobacteria
produce oxygen
Chlamydia
cause chlamydia
Planctomyces
unusual cell shape
Deinococcus
radiotolerant, find in radioactive environments
Green nonsulfur bacteria, Thermotoga, OP2, Aquifex
thermophilic
close to LUCA
Domain bacteria phenotypic diversity within groups/phyla
Many phyla phenotypically diverse, physiology and phylogeny not necessarily linked
Major Archaeal phyla
Euryarchaeota
methanogens
produce methane
extremophiles
Crenarchaeota
most are extremophiles
some live in marine, freshwater and soil systems
Microbes that grow at different temperatures
Psychrophiles
grow optimally at <15 degrees, max temp for growth <20 degrees, min temp <0
psychrotolerant
can grow at 0, optimal 20-40
mesophiles
optimal temp 15-40 degrees, usually 39
thermophiles
optimal growth 45-80
hypethermophiles
optimal growth >80 degrees
Microbes that grow at different pH
acidophiles
optimal growth low pH (<6)
neutrophiles
optimal growth btwn pH 6-8
alkaliphiles
optimal growth high pH (>8)
Microbes that grow in varying levels of salinity
Halophile
optimal growth at 1-15% NaCl
Extreme halophile
optimal growth 15-30% NaCl
Halotolerant
can tolerate some NaCl but grow best in its absence
Microbes that grow in different pressures
barophile
thrives at high pressure
most grow in darkness and highly UV sensitive
obligate barophile
can’t survive outside of high pressure
barotolerant
able to survive at high pressure but can exist in less extreme environments
How have different extremophiles adapted to their environments?
Psychrophiles
higher content unsaturated fatty acids to keep membrane fluid at low temp
Thermophiles
bacteria
rich in saturated fatty acids
archaea
lipid bilayer fused into monolayer, increasing rigidity
Barophiles
higher proportion unsaturated fatty acids keeps membrane from gelling at high pressure
Halophiles
higher proportion of acidic proteins with negative charge prevents salts coagulating proteins
Acidophiles
proteins with higher isoelectric point to keep them stable at low pH (makes them +ve)
simplified electron transport chain
Oxygen and microbial growth
Aerobes
require oxygen to live (21% or higher)
Anaerobes
do not require oxygen
may be killed by oxygen
Facultative organisms
can live with or w/o oxygen
typically grow better in presence of oxygen
Aerotolerant anaerobes
tolerate oxygen even though don’t use it
Microaerophiles
use oxygen at low concs
Toxic oxygen species
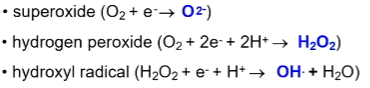
How aerobes deal with toxic oxygen species
toxic oxygen species produced during metabolism are metabolised by catalase into something safe
obligate anaerobes are oxygen sensitive bc they do not contain catalase - cannot detoxify reactive oxygen species
Classes of microbes according to carbon source
autotrophs
use co2 as carbon source (carbon fixation)
heterotrophs
one or more organic molecules, feed on autotrophs or autotroph products
mixotrophs
organic compounds or co2 fixing
Redox tower
represents range of possible reduction potentials in nature
top = greatest tendency to donate electrons
bottom = greatest tendency to accept electrons
difference in redox couple is released as energy
oxygen is at the bottom for electron acceptors, thus more energy released for microbes using oxygen
How is energy conserved (stored in different forms)
Energy released in redox is stored in ATP
long term storage = insoluble polymers like glycogen which can be oxidised to gain ATP
Electron carriers
Shuttle electrons and/or protons
NAD+/NADH, FAD+/FADH
Work done in stages down redox tower, electron carriers are part of the tower (intermediates)
What metabolism needs
Energy
for anabolism - building compounds
from catabolism - breaking down compounds
sources
chemical (chemo-)
organic (-organo-)
inorganic (-litho-)
light (photo-)
Carbon source
co2 (autotrophs)
organic molecules (heterotrophs)
mix (mixotrophs)
Other molecules - nitrogen, phosphorus, sulfur, magnesium, nickel, calcium
Thus naming is chemolithoautotroph
chemolitho = energy from chemicals - specifically oxidation of inorganic compounds
auto = carbon source - co2, fixes into organic compounds
Distinguish between chemoorganotrophs, chemolithotrophs and phototrophs
chemo- vs photo- = get energy from oxidation of chemicals vs light
organo vs litho (subcategories of chemotrophs) = oxidise organic compounds vs inorganic compounds to get energy
chemoorganotrophs - typically heterotrophs (use organic compounds as carbon source)
chemolithotrophs - typically aerobic respiration, most are autotrophs (carbon fixing)
Mechanisms for producing energy
Fermentation
anaerobic process
use organic molecule as both acceptor and donor - only chemoorganotrophs can perform
Substrate level phosphorylation is anaerobic and doesn’t require electron transport chain
but reduces NAD+ to NADH
fermentation reoxidises NADH to NAD+, allowing this to continue
net 2 ATP per glucose
Respiration - requires electron transport chain
anaerobic
lower ATP yield than aerobic, still higher than fermentation
aerobic
oxygen as terminal acceptor
38 ATP yield
Fermentation process
Substrate level phosphorylation
Glycolysis steps (catabolism of sugars)
ATP added to glucose
split into 2× 3-carbon compounds
NAD+ reduced to form NADH
2x compounds further oxidised to pyruvate
pyruvate reduced to lactate or ethanol
uses electrons from NADH, NADH is oxidised
making NAD+, thus pathway can cycle
Respiration process - specifically electron transport chain
Electron transport chain + chemiosmosis
electrons transferred across chain to terminal acceptor, protons from NADH or flavoproteins pumped across membrane
carriers in cell membrane arranged in order of increasingly +ve reduction potential, ending in terminal e- acceptor
proton gradient created, ATP synthase converts proton motive force to ATP
Electron Carriers
NADH dehydrogenases
binds NADH, accepts 2 protons 2 electrons
protons pumped across cell membrane
Quinones (Q)
non-protein containing molecules that participate in electron transport
Flavoproteins and Ubiquinone (Coenzyme Q)
pumps protons across membrane
flavoproteins lose their protons which are pumped across membrane
Cytochromes (cyt)
proteins containing heme groups
accept and donate single electron via iron atom in heme
terminal cytochrome donates electrons to terminal electron acceptor
Major forms of anaerobic respiration
Nitrate (NO3-) reduction and denitrification (nitrate reduction all the way to N2)
denitrification main biological source of nitrogen gas
want to denitrify all the way to N2 (safe)
Nitrate (NO3-) → nitrite (NO2-) → nitric oxide (NO) → nitrous oxide (N2O) → N2
Sulfur oxidation and reduction
Other metals e.g., manganese
Different types of chemolithotrophs
Use different inorganic compounds for oxidising, these are on different positions on the redox tower and generate different amounts of energy, meaning depending on substrate being oxidised chemolithotrophs grow fast or slow
Sulfur oxidisers
many sulfur compounds oxidised
sulfate final product
elemental sulfur can be deposited in cells as granules
Nitrifying bacteria
oxidise ammonia and nitrite
nitrification = ammonia to nitrite to nitrate
only small energy yields so growth of bacteria very slow
2 groups of bacteria to fully oxidise ammonia intro nitrate
nitrosomonas
ammonia → nitrite
inhibited by nitrapyrin - farmers use to keep ammonia in soil longer
nitrobacter
nitrite → nitrate
commamox - can do whole process of nitrification by self
importance of nitrification
nitrate = key nutrient for plants
nitrification key step in waste water treatment - ammonia produced during breakdown of organic matter
nitrate produced by nitrifiers removed by denitrification
iron oxidisers
very small energy yields
oxidise ferrous iron (Fe2+) (soluble) → ferric iron (Fe3+) (insoluble)
Fe2+ is stable only at low pH, thus iron oxidisers are acidophiles
some grow at neutral pH if in anaerobic environment
a lot of iron is oxidised and put through a simple transport chain to keep process going and cytoplasm pH at 6
anoxic vs anaerobic
anoxic = no dissolved oxygen (O2) but can have bound oxygen (e.g., nitrate)
anaerobic = no oxygen at all (not even in bound forms)
Photosynthesis types
Oxygenic
water → oxygen
use light to generate ATP and NADPH
photosystem II
light splits water into oxygen, pushes electrons to high energy, fall down transport chain to photosystem I
photosystem I
electrons pushed up again, flows down chain to NADPH
Z scheme (II to I, graph of reduction potential looks like Z)
ATP produced by
non-cyclic photophosphorylation (II)
cyclic photophosphorylation (I)
electrons can flow back to photosystem II from fd to cyt bf
chlorophyll is the photosynthetic pigment
Anoxygenic
cyclic process
reducing power for CO2 fixation comes from inorganic electron donor (ferrous iron, H2S, NO2) instead of water
oxygen not produced
bacteriochlorophyll
Organisation of photosynthetic pigments in phototrophs
Chl/Bchl
Not in chloroplasts like in plants
In Reaction Centres (RC)
Antenna pigments (LH) funnel light to reaction centres
Chlorosomes function as massive antenna complexes, capturing low intensity light
Carotenoids
accessory pigments always present
energy absorbed transferred to RC
primary role as photoprotective agents, preventing photo-oxidative damage from toxic oxygen species
Citric acid cycle
pathway through which pyruvate is completely oxidised into CO2
initial steps (glucose to pyruvate) same as glycolysis
Provides energetic advantage over fermentation
Metabolic features unique to prokaryotes
Anaerobic respiration
e- acceptors other than oxygen
denitrification
Chemolithotrophy
inorganic energy sources (e.g., nitrification, hydrogen, iron and sulfur oxidisers)
methanogenesis - generate methane from co2 or organic compounds
mehtanotrophy - co2 from methane
nitrogen fixation - nitrogen gas to ammonia
Carbon cycle - oxic and anoxic processes
CO2 to organic matter is done by autotrophs - chemolithoautotrophs and photoautotrophs, autotrophic methanogens
Organic matter to CO2 (decomposition of organic matter) is done by heterotrophs - chemoorganotrophs usually, or methanotrophs, heterotrophic methanogens
Oxic (oxygen present)
CO2 to organic matter
oxygenic photosynthesis
organic matter to CO2
aerobic respiration
anthropogenic activities
methane to CO2
methanotrophy
Anoxic (oxygen absent, but can be in compounds)
CO2 to organic matter
anoxygenic photosynthesis
organic matter to CO2
anaerobic respiration
fermentation
CO2 to CH4 (autotrophic)
methanogenesis (CO2 + H2 → CH4)
organic matter to CH4 (heterotrophic)
methanogenesis (ethanoic acid → CH4 + CO2)
Methanogenesis
Only carried out by archaea
Autotrophic or heterotrophic (using acetate)
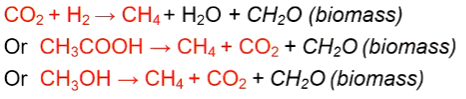
Methanogens role in cow rumen microbiome
In cow rumen
cellulose hydrolysed into sugars
sugars fermented into volatile fatty acids by other bacteria, producing CO2, hydrogen
perfect environment for methanogens (anoxic), they use this hydrogen which could inhibit other hydrolysis enzymes
Permafrost risks
biomass from plants trapped in permafrost
global warming = earlier thawing
ponds become anaerobic, microbes degrade carbohydrates to generate CO2, making an anoxic environment
methanogens convert CO2 and hydorgen into methane which goes into atmosphere, longer growing seasons, more biomass, +ve feedback loop
Nitrogen cycle key processes
Nitrification
ammonia oxidation to nitrate
nitrapyrin inhibits
denitrification
nitrate reduced to nitrogen gas
nitrogen fixation
reduction of N2 to ammonia, making nitrogen biologically usable
ammonification
breakdown of proteins into ammonia
anammox
anaerobic ammonium oxidation
NH4+ + NO2- → N2 + 2H2O
occurs in anammoxosome which protects cell from toxic intermediates (hydrazine)
removes ammonia from marine environments, wastewater
Nitrogen cycle oxic and anoxic processes
Oxic
nitrification
ammonification
Anoxic
denitrification
anammox
nitrogen fixation
ammonification
Nitrogen fixation (process, symbiosis with roots, regulation and importance)
Process
uses nitrogenase (composed of dinitrogenase and dinitrogenase reductase) in redox reactions
sensitive to oxygen
in aerobic cells nitrogenase protected from O2 by
high respiration retes to use oxygen
slime layers
compartmentalisation
oxygen scavenging by leghemoglobin
reduce N2 to ammonia
energy comes from fermentation, photosynthesis, respiration
16-24 ATP for 2 ammonia
Symbiosis with roots
bacterial nodules in plant roots fix nitrogen plants can use
plants provide carbon source (organic acids) used in citric acid cycle for energy
Regulation
Nif genes
highly regulated bc energy intensive process
nitrogenase highly regulated
N fixation blocked by
presence of oxygen
higher concs of ammonia, nitrate
certain amino acids
Importance
fix nitrogen from gas in atmosphere (n sink) to make it biologically usable
Early Earth conditions and early metabolism
Anoxic, hot
early metabolism
anaerobic and chemolithoautotrophic
obtained carbon by fixing co2 to biomass
lithotrophs produced organic carbon, leading to evolution of organotrophs
Surface origin hypothesis and Miller-Urey Experiment
cellular organisms rose out of organic and inorganic primordial soup on surface
temp fluctuation, meteor impacts, dust storms argue against this
supported by Miller Urey Experiment
passed electricity through simple organic compounds (similar conditions to early earth)
formed organic carbon and amino acids
Subsurface origin hypothesis
life originated at hydrothermal springs on ocean floor, stable conditions
conditions for organic molecule generation favourable
slightly acidic, iron rich water
precipitates, geochemical processes to create organic compounds
stages of development for cellular life
cell envelope from montmorillonite clay vesicles
synthesis of phospholipid membrane vesicles
proteins to catalyse reactions rather than spontaneous and metal catalysts
RNA world theory
first self-replicating systems may have been RNA-based
DNA more stable, eventually becomes genetic repository
3 part system developed
DNA
RNA
Protein
Timeline of microbial evolution
Methanogenesis
First phototrophs (anoxygenic)
cyanobacterial lineages used water instead of H2S, generated O2
O2 could not accumulate until reacted with ferrous iron to produce ferric iron, producing banded iron formations
Bacteria and archaea begun to use oxygen (more energetically favourable)
oxic atmosphere - new higher energy metabolic pathways, ozone layer to protect from UV radiation
unicellular eukaryotes
multicellular eukaryotes
Banded iron formations
O2 could not accumulate until reacted with soluble ferrous iron in ocean to produce ferric iron (insoluble), shown by banded iron formations
Led to iron oxide formation
Consequence of O2 for evolution of life
formation of ozone layer
barrier against UV radiation
allowed life to develop above ocean surface