CH. 9 | Chemical Bonds + An Introduction to Chemistry
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Chemistry
The study of matter and the changes it undergoes
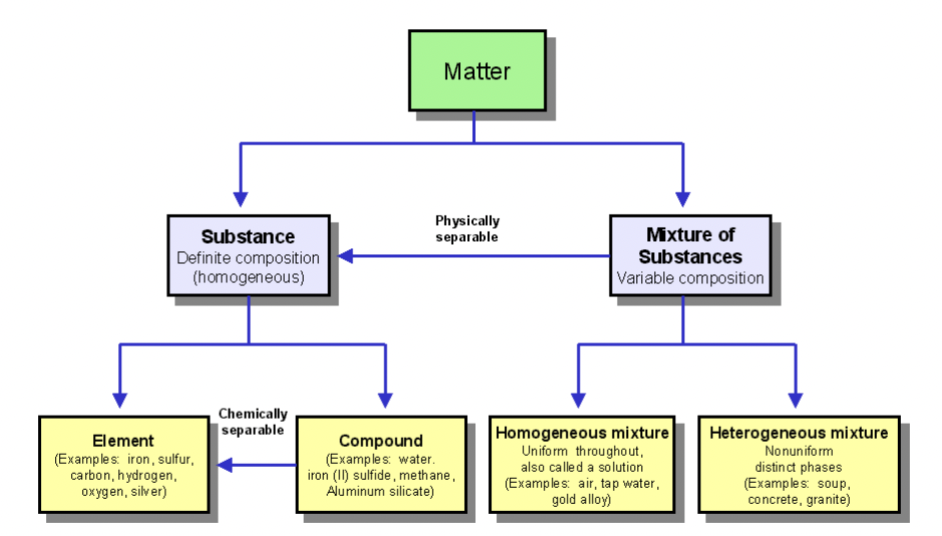
Matter
Anything that takes up space
→ Pure substance
Elements
- Starting material of all matter
i.e., Na , O , C , N , H
Compounds
- Two or more elements chemically bonded together
i.e., H2O
→ Mixtures
Two or more pure substances in the same container
Homogenous mixture
- CANNOT BE SEEN BY THE NAKED EYE
- No boundaries observed
i.e., airHeterogenous Mixture
- CAN BE SEEN
- Boundaries allowed
i.e., smog
States of Matter
Solid (s) , Na
Liquid (l) , Hg
Gas (g) , He
Aqueous (aq) , NaCl
- Dissolved in water
Atom
Smallest building block of matter
Molecule
Larger unit in which 2 or more atoms are joined together
Monatomic, Diatomic, Triatomic
mon/mono → one
di → two
tri → three
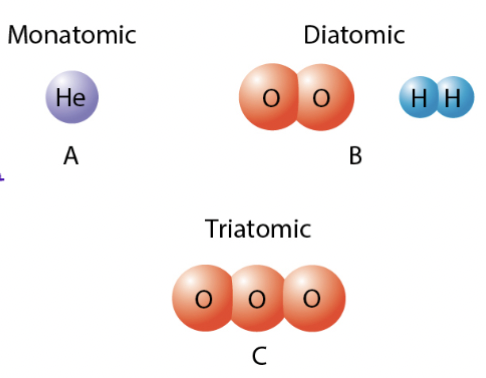
Diatomic molecules
Element composed of 2 atoms
i.e.,
H2 , O2 N2 F2 Cl2 Br2 I2
ALWAYS COVALENT
Chemical Reactions
Formation and/or breaking of chemical bonds to form new molecules (products) from old ones (reactants)
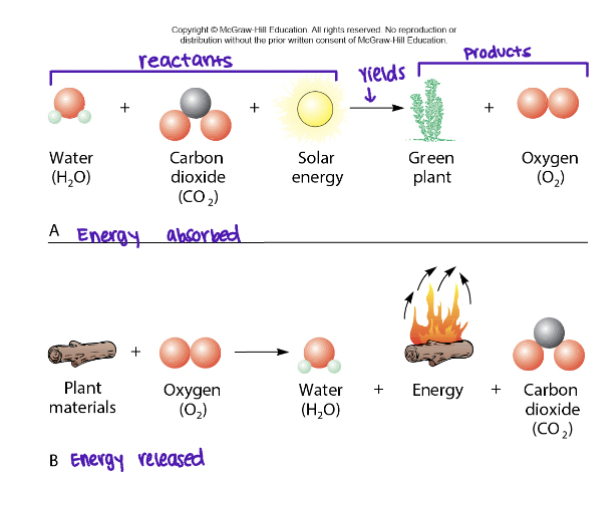
Chemical energy
Internal bonding potential energy
Chemical equation
Symbolic summar
Valence electrons
Outer electrons determining the chemical properties of an atom
Octet rule
Atoms attempt to acquire an outer shell of 8 electrons
Electrons can be gained / lost / shared in the process
BASICALLY, ELEMENTS IN THE P.T WANT TO BE LIKE NOBLE GASES (They already fulfill the octet rule)
Chemical Bonds
Attractive forces holding atoms together in compounds
3 types of Chemical Bonds
Covalent bond
Ionic Bond
Metallic Bond
Energy and Electrons in Ionic Bonding
Reaction energy released = heat of formation
Electron transfer rules
Electrons lost/gained to form closed octets
# gained = # lost
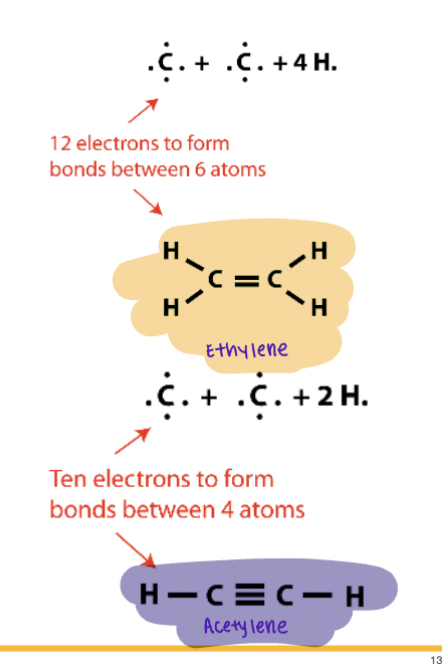
Multiple Bonds
Sharing of more than one electron pair
i.e.,
Ethylene = double bond
Acetylene ≡ triple bond
Bond Polarity
Result of unequal sharing of electrons
Polar covalent bonds
Electronegativity
Measure of an atom’s ability to attract electrons
Ionic & Covalent Compound Names + Formulas
…
6 Polyatomic Ions
OH-1 Hydroxide
NO2-1 Nitrite
NO3-1 Nitrate
CO3-2 Carbonate
SO4-2 Sulfate
PO4-3 Phosphate