Evaluating health behaviour change interventions via experiments and randomised controlled trials (RTC)
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
Qualitative data
non numerical data
focus on why and how
generates theories based on data
'data are analysed via categorisation
quantitative data
numerical data, to quantify variables and for statistical estimation or inference
focus on what and how much
test theories and hypothesis
data are analysed using statistical methods
quantitative behaviour is used if you;…
want to quantify behaviours
establish general patterns
test specific theories or hypothesis
qualtitative behaviour is used if you;…
explore new research areas or phenomenon
understand deep psychological processes
study individual experiences
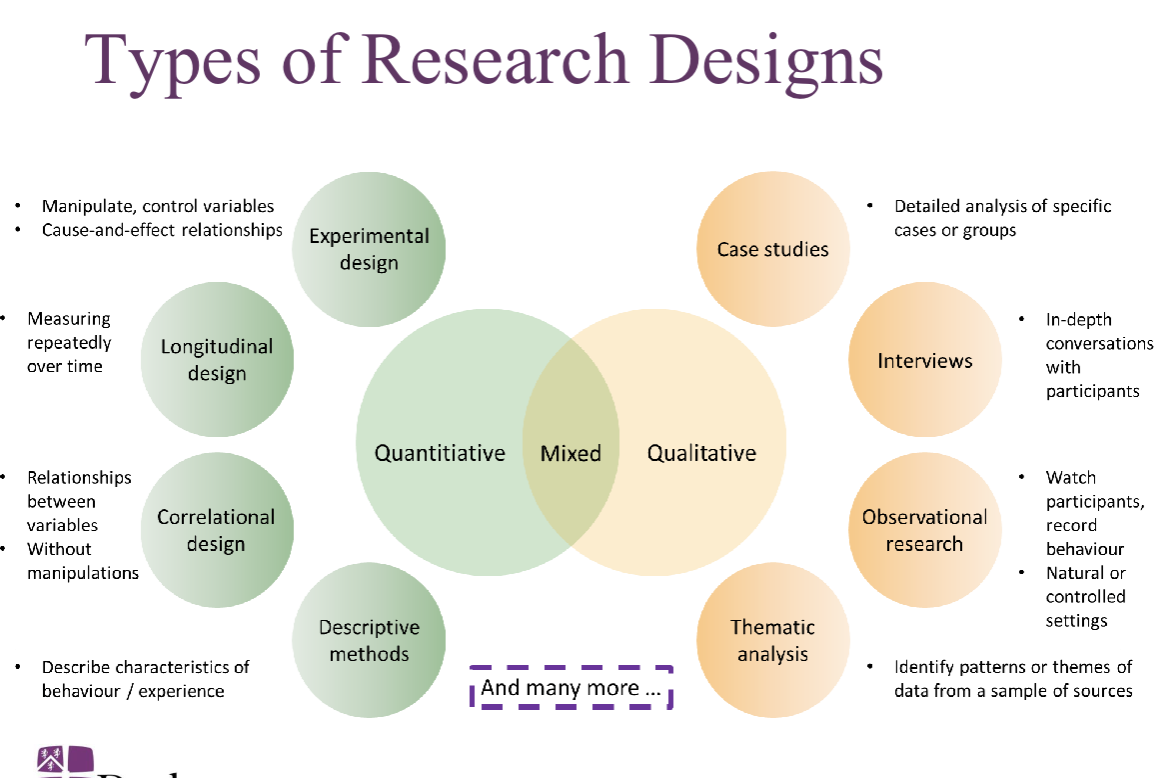
Types of research design
Extraneous variables are
any variables other than the IV that may affect the DV
extraneous variables become confounding variables when…
their values change systematically with the level of the IV
The impact of extraneous and confounding variables is assumed to be …
minimised through ‘true’ randomisation
What are RTCs
RTCs are a subset of possible experimental design
an RTC is a planned experiment designed to assess the efficacy of an intervention by comparing the intervention group to a control group
the allocation to intervention or control group is determined purely by chance (true randomisation)
True (proper) randomisation in experiments/ RTC’s
eliminates bias in treatment/ intervention assignment
ensures that differences between experimental groups e.g. intervention vs control group can be attributed to the treatment/intervention
Permits the use of probability theory to express the likelihood that any differences in outcome between treatment/ intervention groups merely indicates choice
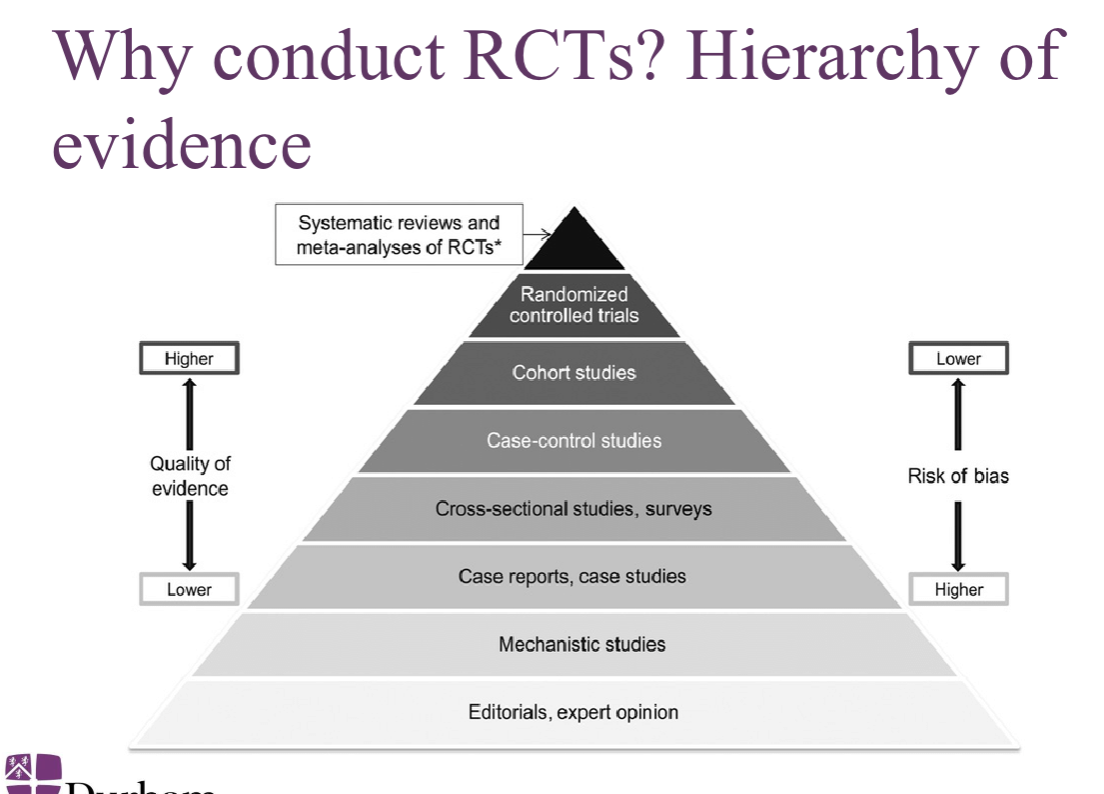
Why conduct RCTs?
observational and quasi experimental deign are subject to potential bias and confounding due to: self selection, observer bias, secular trends (before and after the study)
RCT provides the gold standard for proof of concept
Why conduct RCTs?
Eligibility criteria
ii. Specified hypotheses
iii. Predefined intervention and control groups (proper control group)
iv. Primary and secondary outcomes/endpoints (e.g., behavioural change, HIV incidence) to address hypotheses
v. Methods for enrolment and follow up
vi. Rigorous monitoring
vii. Analysis plans and stopping rules
viii. Comprehensive reporting of methods and data analysis
Why conduct RCTs? hierarchy of evidence
The history of RCT’s -
First ever RCT conducted in 1747 by James Lind examining the impact of citrus in treating scurvy
First published RCT in medicine “streptomycin treatment of pulmonary tuberculosis”, which described a medical research council (MRC) Investigation conducted by Austin Bradford Hill
Types of RCT’s - Individually randomised trials
Eligible individuals are randomised (conventional medical RCTs and
many behavioural RCTs)Self-selection of persons volunteering for enrolment
Types of RCT’s - cluster randomised trials
Clusters (e.g., communities, hospitals), or other aggregates of people
(e.g., workplaces, bars) are randomised, and all consenting persons
enrolledLess individual-level self-selection -> increasing generalisability
Nature of the intervention (e.g., mass media campaign, population-level
interventions)Acceptability and reduced stigma (everyone gets the same treatment within a cluster)
Types of RCT’s - cluster randomised trial - BUT
Cluster randomisation more vulnerable to lack of comparability between
study groups than individual randomisation (fewer units of
randomisation, more correlated characteristics within members of
clusters)Cluster RCTs increase sample size requirements and are less efficient
than individual RCTs due to intra-cluster correlation
The different steps taken when designing and implementing RCTs - pic

Steps taken when designing and implementing RCTs - 1- conduct a sample size calculation
a sample size calculation is an a priori statistical analysis to determine the appropriate number of participants needed in the RCT in order to detect a meaningful statistical effect
an effect size is the magnitude of the differences between 2 groups (arms/conditions within the RCT
Steps taken when designing and implementing RCTs - 1- conduct a sample size calculation - 1a - sample size calculation for individually randomised RCTs
specific type 1 and 2 error (e.g. power less than 80%) to detect a difference significant at p - 0.05
specific an expected or meaningful difference in outcome rates between intervention and control groups
Estimate losses to follow up on the primary outcome
Estimate required sample size at enrolment using conventional formula
Steps taken when designing and implementing RCTs - 2 - register the protocol for the RCT - protocol contains precise information regarding
intervention vs. control groups [includes information on what exactly the
different groups are exposed to]Outcome/endpoint measures (what we know as DVs in experiments)
Hypotheses
Participant eligibility criteria
Analysis plan
Steps taken when designing and implementing RCTs - 2 - register the protocol for the RCT - overviews
fulfil ethical oblicagtiosn to participants and researcher
provide information to potential participants and referring clinicians
reduce publication bias
help editors and others understand the context of study results
promote more efficient allocation of research funds
Help institutional review boards determine the appropriateness of a research study
Steps taken when designing and implementing RCTs - 2 - register the protocol for the RCT - control groups
Controls may receive no treatment/intervention (e.g., placebo, or
nothing in behavioural RCTs) if there is no standard of care (no
previously accepted intervention that works)If there is an established standard of care it would be unethical to
withhold this from controls, so standard of care becomes the reference
control
Steps taken when designing and implementing RCTs - 2 - register the protocol for the RCT - Primary outcome
Primary outcome is the outcome that an investigator considers to be
the most important among the many outcomes that are to be examined
in the study.The primary outcome needs to be defined at the time the study is
designed.The primary outcome reduces the risk of a Type I error resulting from
the statistical testing of many outcomes.It reduces the risk of a Type II error by providing the basis for the
estimation of the sample size necessary for an adequately powered
study.
Steps taken when designing and implementing RCTs - 2 - register the protocol for the RCT - Secondary outcome
Secondary outcome measures, also known as secondary endpoints,
may provide information on therapeutic effects of secondary
importance, side effects, or tolerability.An operational definition (operationalisation) is how a variable is
measured [and, this forms an important part of the registration].
Steps taken when designing and implementing RCTs - 3 - enrolment - select and define study sample - elibility criteria
Eligibility criteria establish the parameters for determining who is able to
participate (inclusion) and who is not able to participate in the study
(exclusion
Steps taken when designing and implementing RCTs - 3 - enrolment - select and define study sample - eligibility is predefined to
Ensure that participants meet the criteria for the intervention (e.g., have
a specific disease for a therapeutic trial, are free of disease for a
preventive trial etc.)Usually eligibility is also defined by age, gender, race, state of health
(absence of contraindications etc.)The narrower the eligibility criteria, the less generalisable the results
Participants must consent to screening for eligibility
Steps taken when designing and implementing RCTs - 3 - enrolment - select and define study sample - enrolment occurs only after
edibility is established, participants only enrolled after providing informed consent
Steps taken when designing and implementing RCTs - 4 - Baseline assessment
assess all participant enrolled in the study on measure pertaining to primary and secondary outcomes and mediating variables prior to introduction or intervention/ treatment
Create a logic model depicting the hypothesised pathway that you are testing
Steps taken when designing and implementing RCTs - 5 - random allocation (randomisation) - There are two main features to consider when randomising participants to study conditions:
implementing a valid randomisation procedure;
establishing procedures to safeguard the integrity of the randomisation
procedure so that unintentional or intentional biases do not influence the
participant allocation process.
Steps taken when designing and implementing RCTs - 5 - random allocation (randomisation) - 5a - implementing a valid randomisation procedure
Simple randomisation: Analogous to a repeated fair coin tossing
Restricted randomisation or Blocking: Done to ensure equal balance of
participants across groups throughout all portions of the study
▪ For example, blocks of six would have 3 intervention/3 control ppsStratified randomisation: Individuals are identified based on important
covariates (sex, age, etc.) and then randomisation occurs within the
strataDynamic or adaptive methods (not common): Not pre-defined, only first
participant truly randomly assigned
Steps taken when designing and implementing RCTs - 5 - random allocation (randomisation) - 5b - safeguarding integrity of randomisation
Concealment of allocation strategies are designed to mask participants’
knowledge about their group assignment (e.g., sealed opaque
envelopes).Prior to beginning study enrolment, the main investigator or RCT
statistician generates the allocation sequence. This pre-generated
sequence must be adhered to when randomising all participants. This
sequence is not shared with other members of the RCT team.
Steps taken when designing and implementing RCTs - 6 - implementing the study groups/ conditions as per protocol - binding is done to minimise participant or researcher bias
Single blinding (researcher but not participant knows the
randomisation group, e.g., some cluster-level RCTs)Double blinding (neither researcher nor participant know the group of
randomisation)Triple-blinding (researcher, participant, and statistician analysing data
from the study all do not know the group randomisation)Unblinded/open (cannot conceal randomisation, e.g., surgical
interventions)
Steps taken when designing and implementing RCTs - 6a - concealment of allocation vs blinding
Concealment of allocation: Procedure to protect the randomisation
process before the subject enters the RCT (Concealment of allocation is ALWAYS feasible)
Blinding: Masking of the treatments after randomisation (once trial
begins)
▪ Blinding is not always feasible
Steps taken when designing and implementing RCTs - 6b - ensuring fidelity to protocol
Throughout the RCT the research team needs to measure and record
the fidelity to protocol, i.e., the extent to which the outcome measures
and intervention are administered in accord with the registered protocol.Any violations to protocol need to be recorded and reported in the RCT
publication (with sensitivity analyses conducted for deviations to
protocol).
Steps taken when designing and implementing RCTs - 7 - follow up
Follow up is conducted at predetermined intervals needed to detect the
occurrence of RCT outcomes/endpointsThe frequency and duration of follow up will depend on:
Type of outcome/endpoint (e.g., response to treatment, development of
new disease, progression of disease, behavioural change, sustainability
of change)The level of risk (e.g., higher the risk, more frequent the follow up)
Steps taken when designing and implementing RCTs - 7 - follow up - Losses to follow up must be minimised because:
Losses are often selective (e.g., high risk persons, low socio-economic
status participants drop out of trials) and this introduces biasLosses to follow up should be comparable in the intervention and
control groups to avoid biased comparisons, if not this leads to attrition
biasLosses to follow up reduce study power by reducing the person-time of
observation
Steps taken when designing and implementing RCTs - 8 - Analysis - intention to treat
Analyse all persons randomised, even if some do not receive the
intervention/control treatment or drop out before completion of RCTAnalysis based on the group participants were initially (and randomly)
allocated toLeast biased and most conservative
Steps taken when designing and implementing RCTs - 8 - Analysis - as treated (per protocol)
Analyse only those who actually complete the RCT
Analysis includes only those participants who completed the
intervention/control group they were originally allocated toPotentially biased by selection of the most compliant and often lowest
risk population
Steps taken when designing and implementing RCTs - Reporting RTC findings
To assist in reporting of RCTs, a well-articulated, structured format (a
checklist) has been developed by the CONSORT (Consolidated
Standards of Reporting Trials) group.
Steps taken when designing and implementing RCTs - Reporting RTC findings - A CONSORT flow diagram -
graphically presents the progress of participants through the different phases of a randomised controlled trial (RCT).
Are results of RCTs always valid?
RCTs can provide conflicting results (so, important to carry out
systematic reviews and meta-analyses)RCT design, execution, analyses, and reporting can be flawed
Intervention vs. control comparisons are internally valid, but restrictions
on participant eligibility can reduce external validity (e.g., specific age or
sex groups omitted)RCTs could suffer from conflicts of interest (e.g., industry funding)