Chemical Kinetics and Stability of Dosage Forms
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
71 Terms
Why is chemical degradation bad for drugs?
Leads to loss of active product
Loss of potency
Potentially toxic degradation products
Why is drug stability important?
Because drug degradation reduces the amount of active drug and can change its properties, leading to reduced solubility and reduced bioavailability
What does chemical kinetics tell us?
How a drug degrades and how formulations can be stabilized.
What are the most common mechanisms of drug degradation?
Hydrolysis
Oxidation
Other pathways (photolysis, isomerisation)
What conditions can catalyse hydrolysis?
Acid (H⁺) or base (OH⁻) catalysis.
What factors can catalyse oxidation reactions?
UV light, heat, and trace metal ions.
How can oxidation be minimised in formulations?
By
using antioxidants
and appropriate packaging (e.g. light-protective containers).
What key stability questions must be answered during formulation?
What is the mechanism of degradation?
What are the products and are they safe?
How stable is a drug in a given formulation?
At what temperature should it be stored?
Should it be stored in a dark place?
What packaging?
What is its shelf life?
Name the routes of degradation
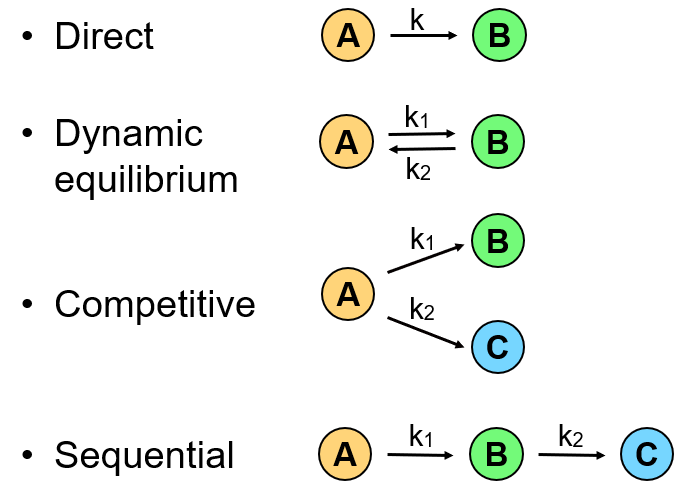
What is molecularity of a reaction?
The number of reactant molecules or ions involved in the rate-determining step.
Define unimolecular, bimolecular, and termolecular reactions.
Unimolecular: one reactant molecule
Bimolecular: two reactant molecules
Termolecular: three reactant molecules (rare in solution)
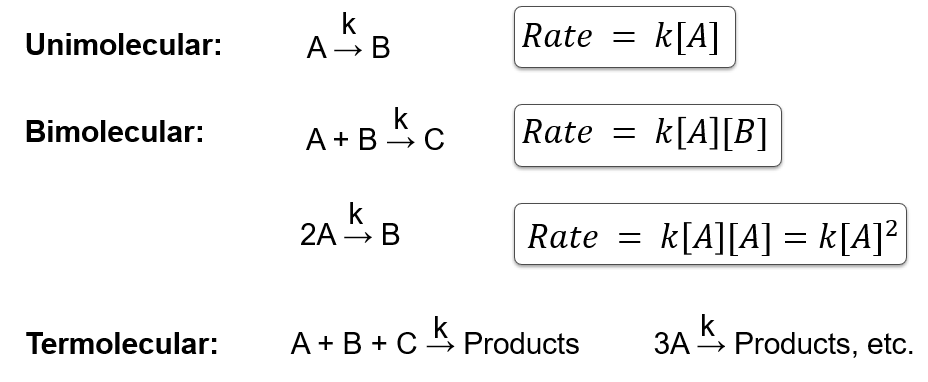
What is the Law of Mass Action?
The rate of a chemical reaction is proportional to the product of the molar concentrations of the reactants, each raised to a power equal to the number of molecules involved.
What is the order of a reaction?
The sum of the powers of the reactant concentrations in the rate equation.
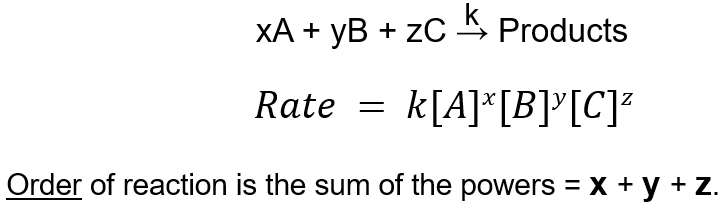
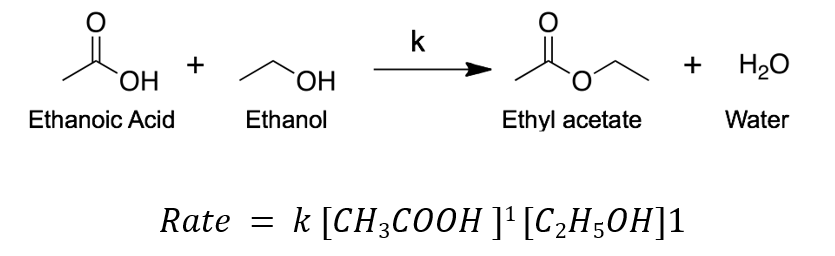
What’s the order of this reaction?
Second order
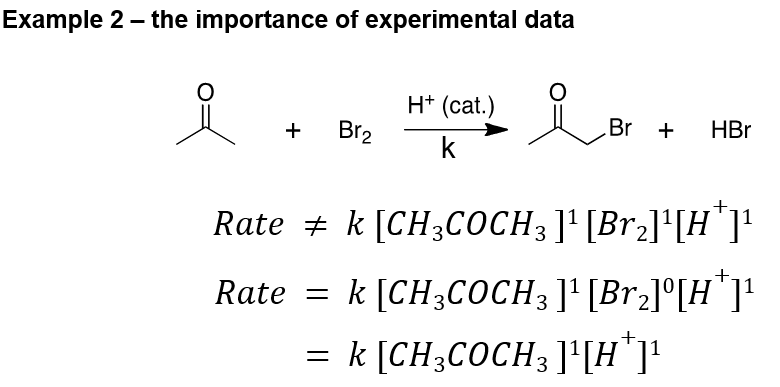
What’s the order of this reaction?
Second order
How does molecularity differ from reaction order?
Molecularity is theoretical (based on mechanism), while order must be determined experimentally.
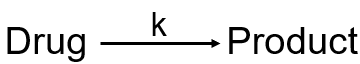
Write the rate equation for a first order reaction.
Rate = k[Drug]
What is the integrated first order rate equation?
ln[Drug] = ln[Drug]₀ − kt
How is first order data plotted graphically?
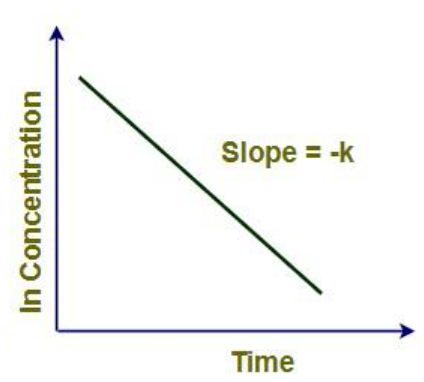
Graphical form of 1st Order Data
[Drug] against Time
![<p>[Drug] against Time</p>](https://knowt-user-attachments.s3.amazonaws.com/b43ff36a-5188-4785-a2a6-d20dda241cdb.png)
How to calculate gradient?
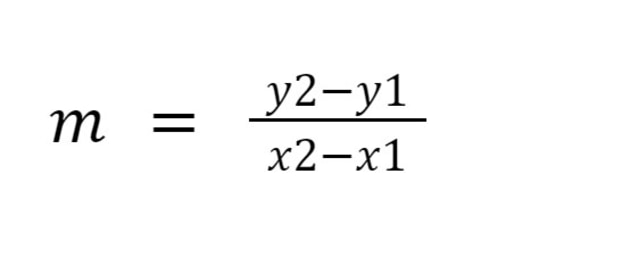
What are the units of the rate constant for a first order reaction?
Time⁻¹ (e.g. s⁻¹, day⁻¹).
What is the half-life (t½) of a first order reaction?
t½ = 0.693 / k
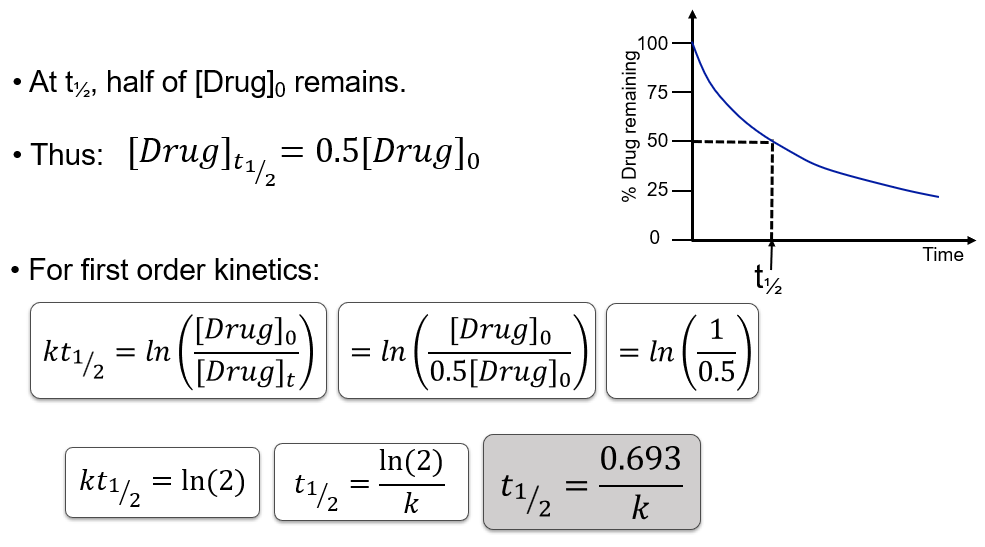
Why is first order half-life independent of initial concentration?
Rate ∝ concentration
A constant fraction is eliminated per unit time,
Every half-life, 50% of whatever is there is removed
It doesn’t matter if you start with 100 mg or 10 mg
→ it always takes the same time to remove halfSo each half-life is the same.
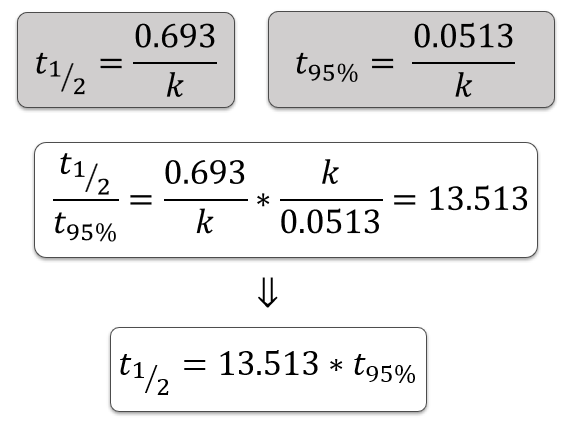
Define pharmaceutical shelf life (t95%).
The time at which 95% of the labelled drug amount remains.
Shelf life (t95%) is the time at which 95% of the API remains.
[Drug]95% = 0.95[Drug]0
Write the shelf life equation for a first order reaction.
t95% = 0.0513 / k
First Order Kinetics – Ratio of t½ to t95%
List common mistakes in first order kinetic calculations.
Using log₁₀ instead of ln
Using raw data instead of best-fit line
Incorrect or missing units
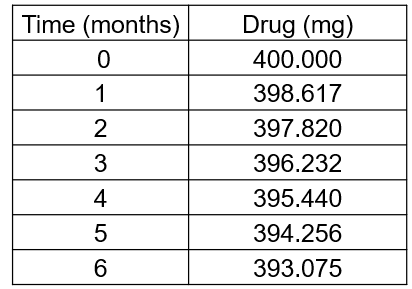
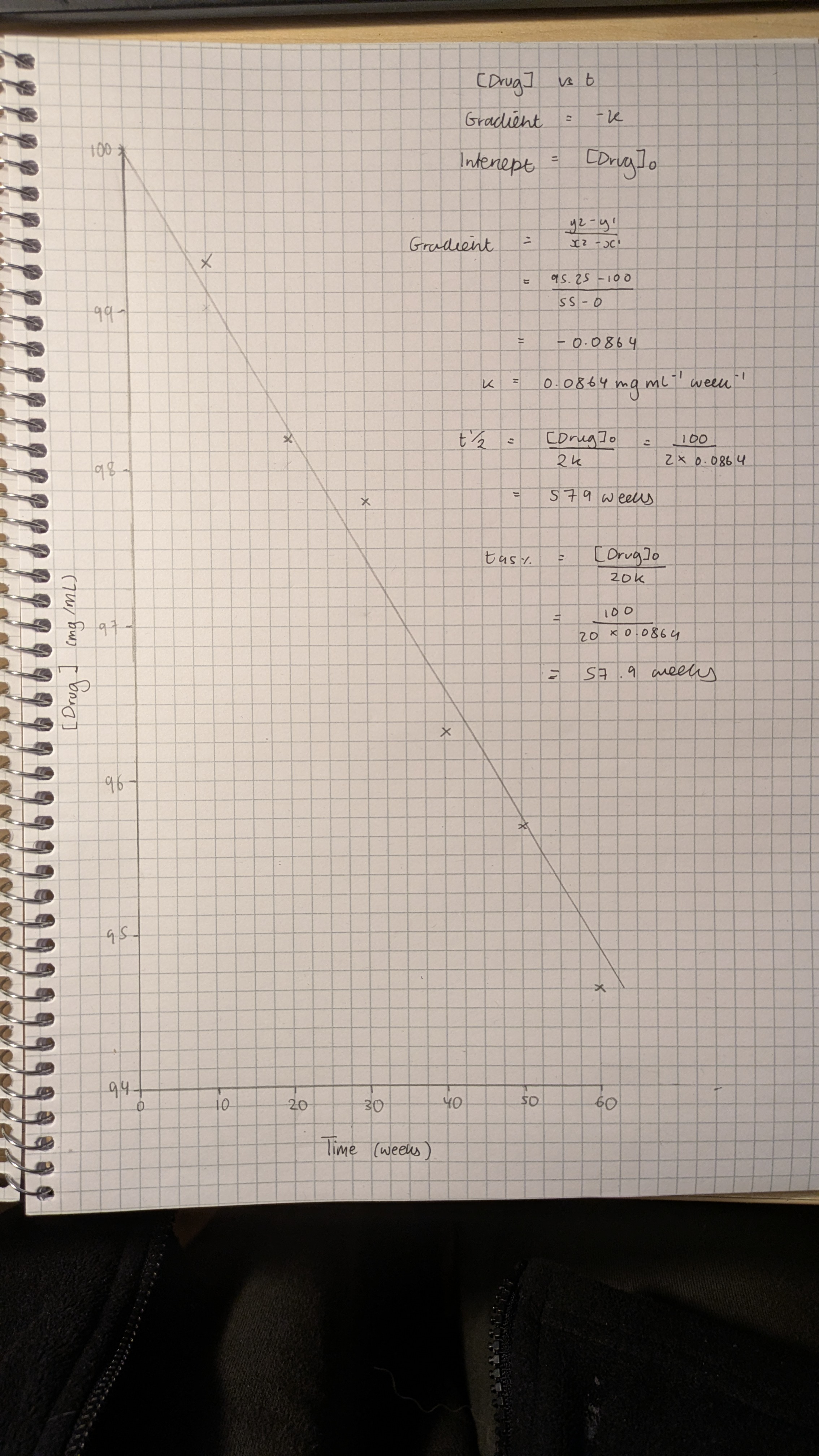
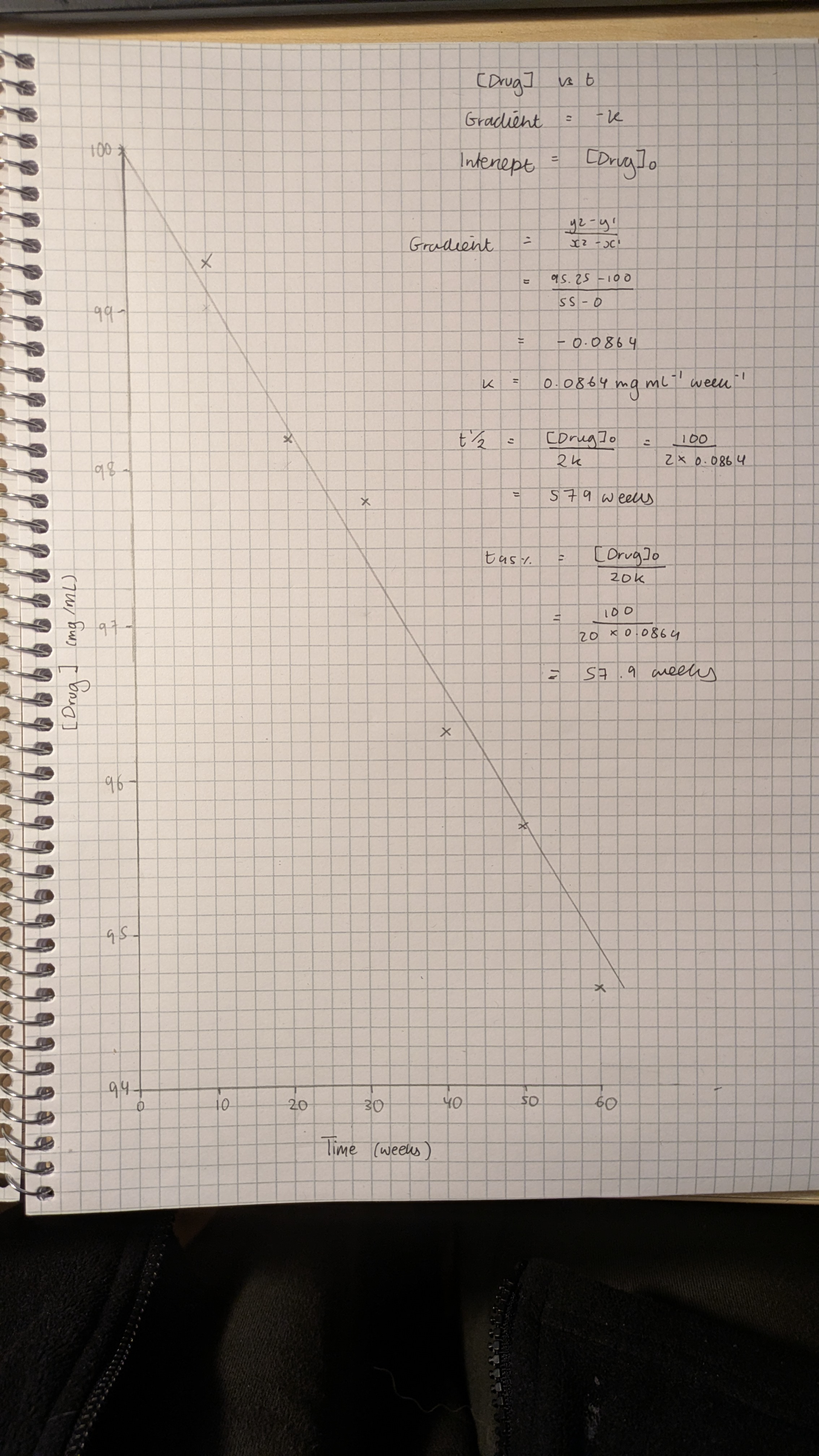
Stability testing of a candidate drug formulation at 25 °C and 60% relative humidity gave the following first order degradation data:
Use this data to plot a suitable graph and use your graph to determine the rate constant for this reaction.
What is the half-life and shelf life of this drug formulation under these standard conditions?
• Calculate ln values from the data
• Plot ln of drug concentration versus time on linear graph paper
• Calculate rate constant k from gradient (m = -k)
• Calculate half-life (t½ = 0.693 / k)
• Calculate shelf life (t95% = 0.0513 / k)
What is zero order kinetics?
The rate of reaction is constant and independent of drug concentration.
Why do pharmaceutical suspensions often show zero order kinetics?
Only dissolved drug can degrade.
As it degrades, more drug dissolves from the solid phase to replace it, keeping the dissolved concentration constant.
A constant concentration gives a constant degradation rate (zero-order).
Write the rate equation for a zero order reaction.
Rate = k
What is the integrated zero order rate equation?
[Drug] = [Drug]₀ − kt
How is zero order data plotted graphically?
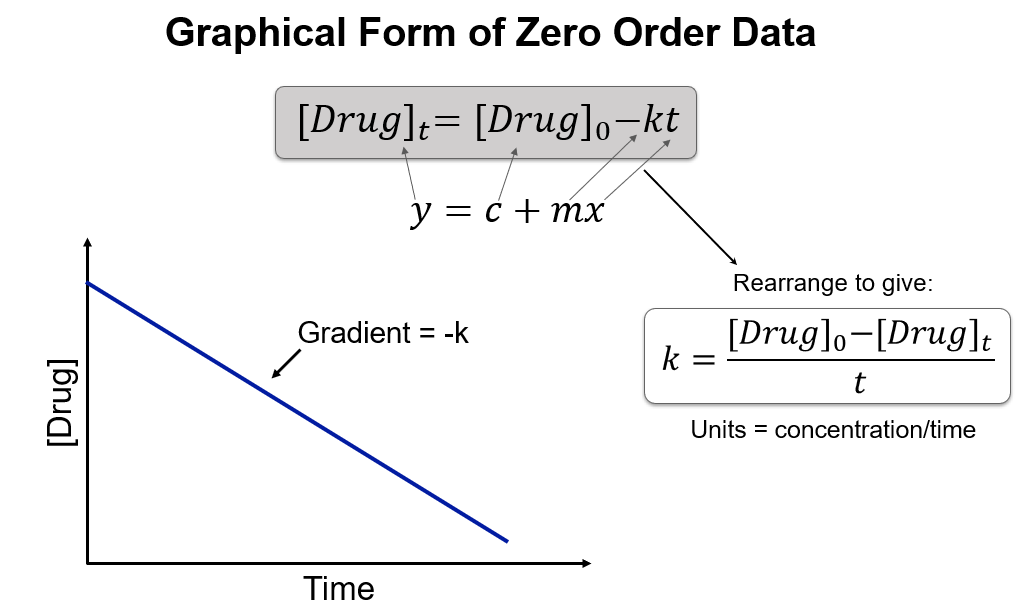
What are the units of k for a zero order reaction?
Concentration per time (e.g. mol L⁻¹ s⁻¹).
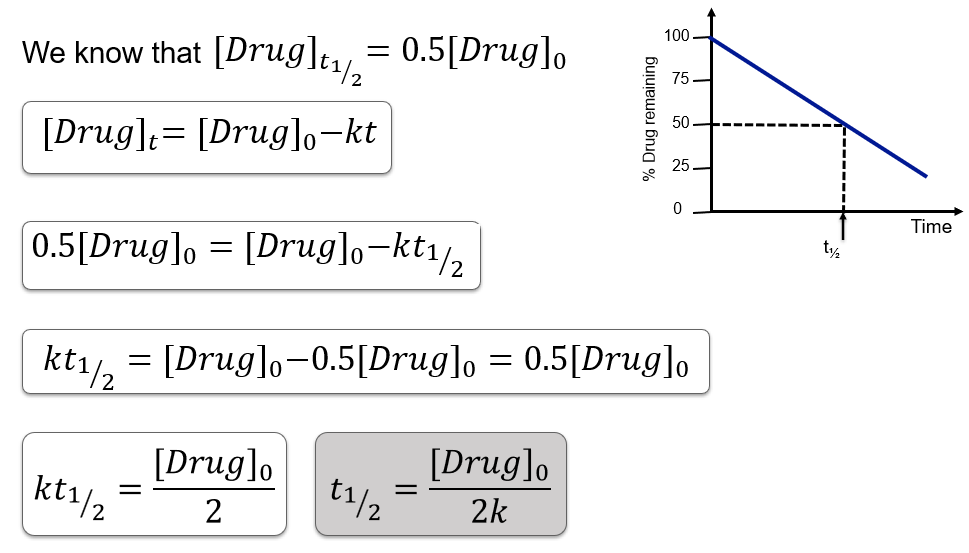
Half-life in zero order kinetics
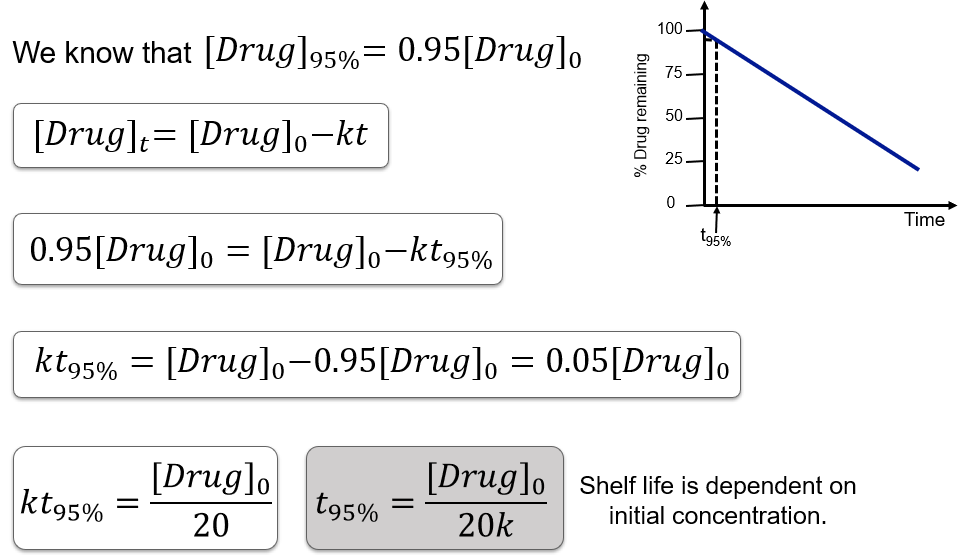
Shelf-life (t95%) in zero order kinetics
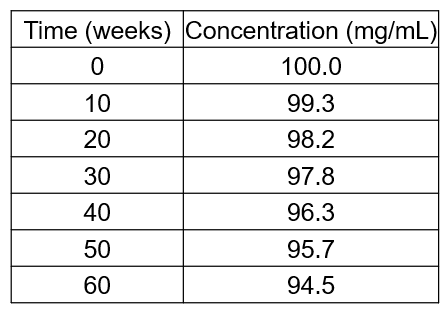
The degradation of an ibuprofen suspension under ambient conditions proceeds as follows:
Use this data to plot a suitable graph and use your graph to determine the rate constant for this reaction.
What is the half-life and shelf life of this drug formulation under these standard conditions?
Why does zero order behaviour eventually stop in suspensions?
Once all solid drug dissolves, the system no longer maintains constant concentration.
How does shelf life differ between first and zero order reactions?
First order: shelf life independent of initial concentration
Zero order: shelf life dependent on initial concentration
Why must reaction order be determined experimentally?
Because real systems often involve complex mechanisms that cannot be predicted theoretically.
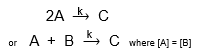
What is a second-order reaction?
Rate ∝ [A]²
Rate ∝ [A][B]
If you double the conc, the rate increases by x4
What type of reactions are described by second order kinetics?
Bimolecular reactions where two molecules collide to form products.
Write the rate equation for second order kinetics when [A] = [B].
Rate = k[A]²
Rate ∝ [A]²
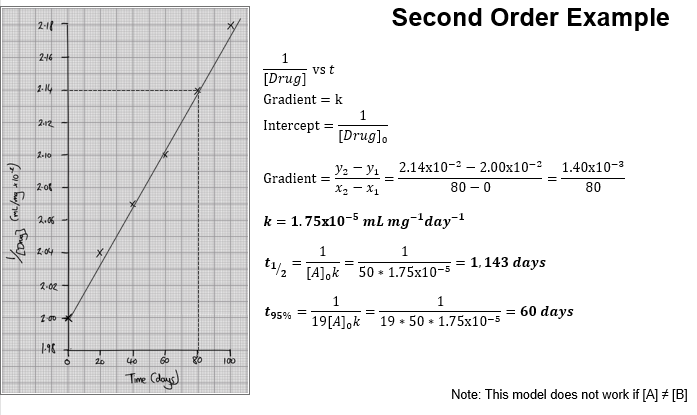
How do you graph second-order data?
Plot 1/[Drug] against time
There is a straight line
Gradient = k (k is rate constant)
![<p>Plot 1/[Drug] against time </p><p>There is a straight line </p><p>Gradient = k (k is rate constant)</p>](https://knowt-user-attachments.s3.amazonaws.com/bab042db-b5f6-4d92-8db2-be07c0e2dec2.png)
What are the units of the rate constant (k) for second order reactions?
Concentration⁻¹ · time⁻¹ (e.g. L mol⁻¹ s⁻¹)
What is the half life calculation in 2nd order kinetics?
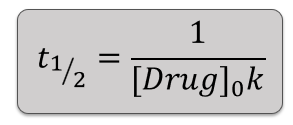
How does half-life behave in second order kinetics?
Half-life depends on the initial concentration.
How does shelf life (t95%) behave in 2nd order kinetics?
Shelf life is dependent on initial concentration.
Increasing [Drug]₀ decreases shelf life.
![<p>Shelf life is dependent on initial concentration.</p><p>Increasing [Drug]₀ <strong>decreases</strong> shelf life.</p>](https://knowt-user-attachments.s3.amazonaws.com/63cdefd2-48db-4836-90b8-a578dd5f00b2.png)
2nd Order Kinetics – Ratio of t½ to t95%
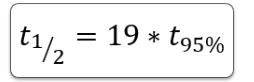
How does increasing initial drug concentration affect t95% in second order reactions?
Increasing [Drug]₀ decreases t95%.
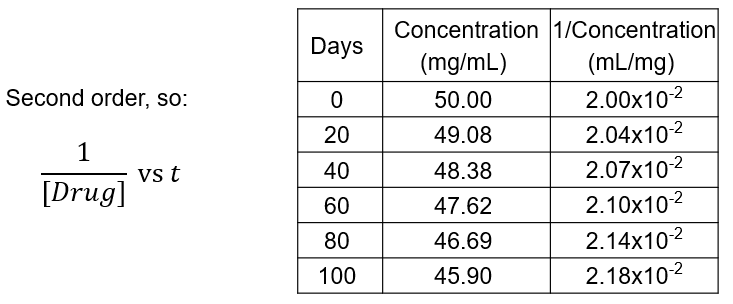
A liquid formulation of a drug for IV injection gives the following second order degradation data when stored at 20 °C:
Plot this data using a suitable graph and use the graph to determine the rate constant for the degradation of this drug.
Calculate the half-life and shelf life of the drug under these conditions.
Name three methods for determining reaction order experimentally.
Substitution method
Shelf life method
Graphical method
What is the substitution method?
The degradation data is substituted into zero, first, and second order equations to see which gives a constant k.
What is the shelf life method?
0 order: Increasing the initial drug conc should increase the shelf life
1st order: The shelf life is independent of the initial drug conc. Increasing or decreasing the initial drug conc doesn’t change the shelf life
2nd order: Increasing the initial drug conc decreases the shelf life

How can plotting data as first order help identify reaction order?
0: [A] vs t linear
1st: ln[A] vs t linear
2nd: 1/[A] vs t linear
![<ul><li><p>0: [A] vs t linear</p></li><li><p>1st: ln[A] vs t linear</p></li><li><p>2nd: 1/[A] vs t linear</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/6333326a-3693-4f8a-ad82-b4d0fc2a4782.png)
What is the effect of ionic strength on rate?
Electrolytes are often added to drug solutions
Inert ions can affect the rate of drug degradation. This is called the salt effect.
Rate can also be affected by the concentration of ions such as H+ or OH-
What is the effect of pH on rate?
pH has a significant effect on the apparent rate constant for many reactions
Hydrolysis is often catalysed by H+, or OH-. This is acid catalysis or base catalysis
Buffering may protect drugs from acid catalysis or base catalysis
Why does increasing temperature increase reaction rate?
Because then more molecules exceed the activation energy
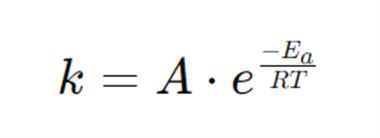
What does everything stand for?
k = Rate constant
A = "Arrhenius factor“, more often known as the pre- exponential factor or the frequency factor
Ea = Activation energy
R = Universal gas constant (8.314 J mol-1 K-1)
T = Absolute temperature (K)
What is an Arrhenius plot?
lnk against 1/T
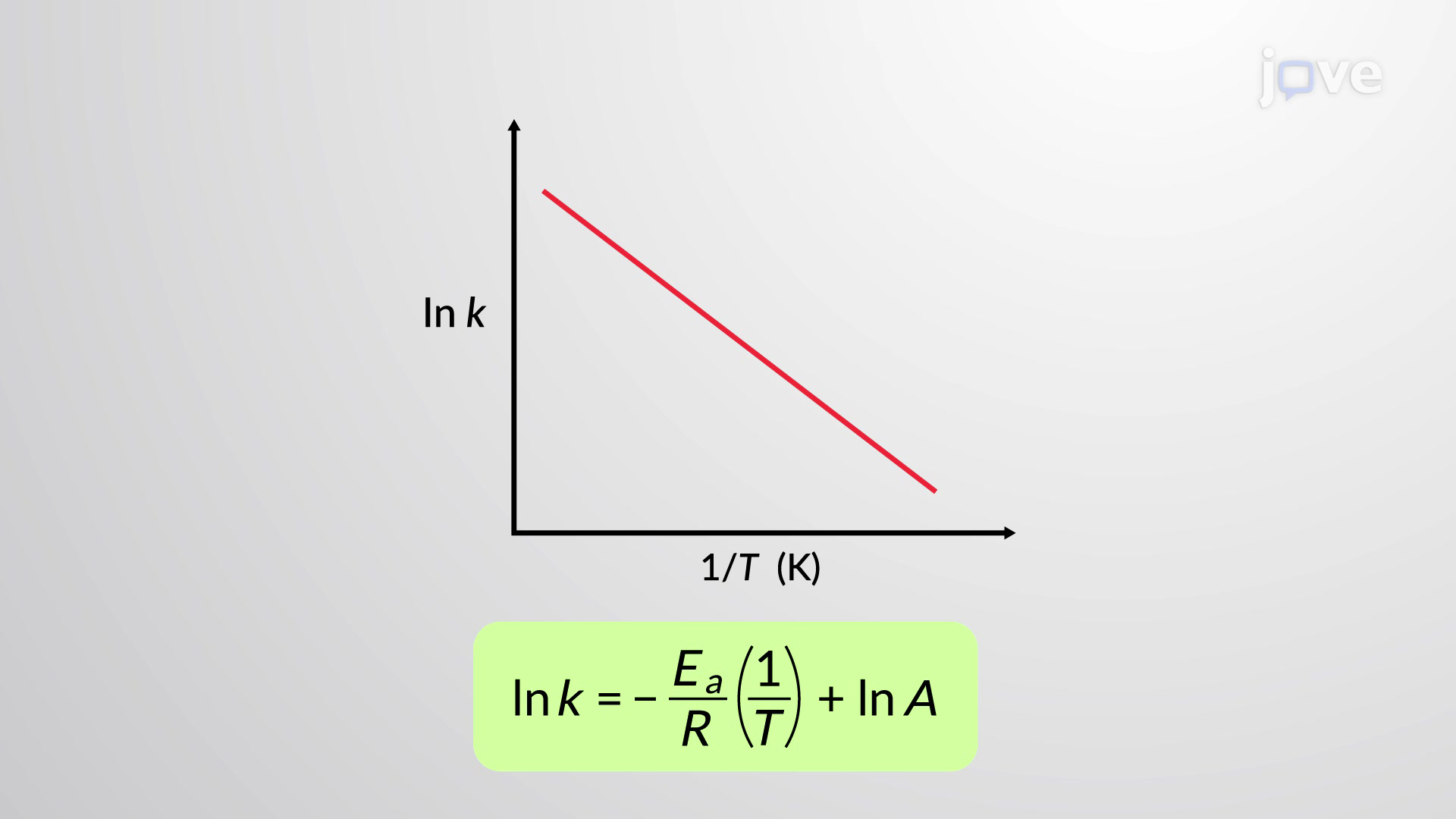
y=mx+c in Arrhenius plot
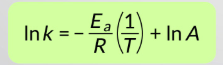
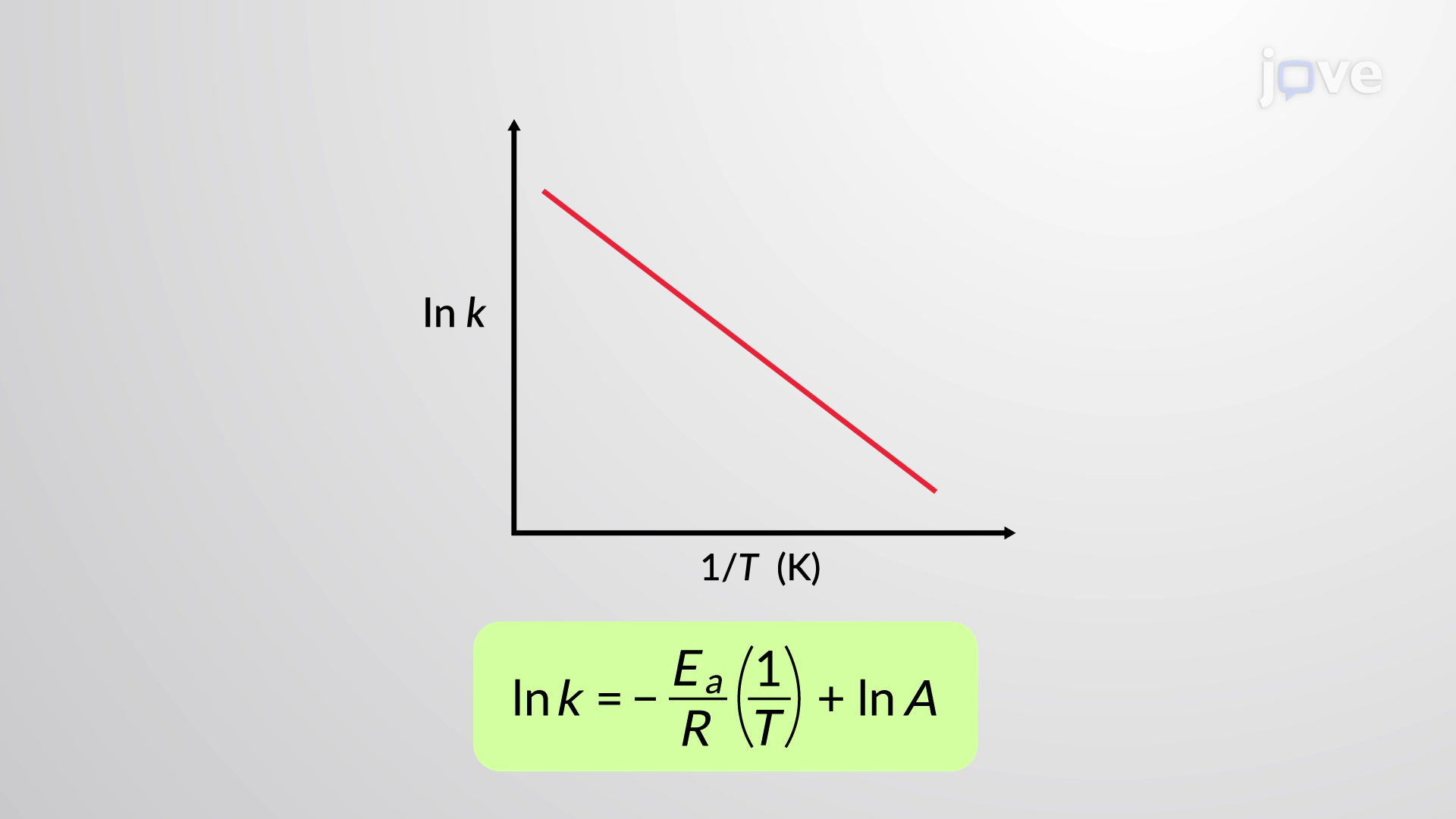
What does the gradient of an Arrhenius plot equal?
−Ea / R
What does the y-intercept (c) of an Arrhenius plot give?
ln A
Why is stability testing important?
Determines storage conditions and shelf life.
What is accelerated stability testing?
Results need to be thoroughly examined.
By using accelerated stability testing, we can estimate the use-by date (with only a few weeks of data)
Instead of waiting years to see how a drug degrades at room temperature, we store it at high temps to make it degrade faster
What is the assumption accelerated stability testing?
The degradation reaction mechanism stays the same across temperatures.
BCS Class I
High solubility
High permeability
BCS Class II
Low solubility
High permeability
BCS Class III
High solubility
Low permeability
BCS Class IV
Low solubility
Low permeability