1 - States of Matter
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Solids
Strong forces of attraction between particles which holds them close together in fixed positions to form a regular lattice arrangement.
The particles vibrate about their fixed positions.
Liquids
Weak forces of attraction between the particles. They are randomly arranged and are free to move but tend to stick closely together.
Liquids have a definite volume but no definite shape.
The particles are constantly moving with random motion.
Gases
Very weak forces of attraction between the particles. They are free to move and are far apart.
The particles constantly move very fast with random motion.
Gases either expand when heated or their pressure increases.
Interconversions between the three states of matter
When a solid is heated, its particles gain more energy.
This makes the particles vibrate more which weakens the forces that hold the solid together. This makes the solid expand.
At a certain temperature, the particles have enough energy to break free from their positions. This is called melting and the solid turns into a liquid
(Freezing is the opposite of this)
When a liquid is heated, the particles gain more energy.
This energy makes the particles move faster which weakens the bonds holding the liquid together.
At a certain temperature, the particles have enough energy to break their bonds. This is called evaporating or boiling and the liquid turns into a gas.
(Condensing is the opposite of this)
Diffusion
The movement of particles from an area of high concentration to an area of low concentration.
Experiments to demonstrate diffusion
Potassium Manganate(VII) and water
Ammonia and Hydrogen Chloride
Bromine Gas and Air
Potassium Manganate(VII) and water
Potassium Manganate(VII) is bright purple, so it is great for this experiment.
If you take a beaker of water and place some potassium manganate(VII) at the bottom, the purple colour slowly spreads out to fill the beaker.
The particles of potassium manganate are diffusing out among the particles of water.
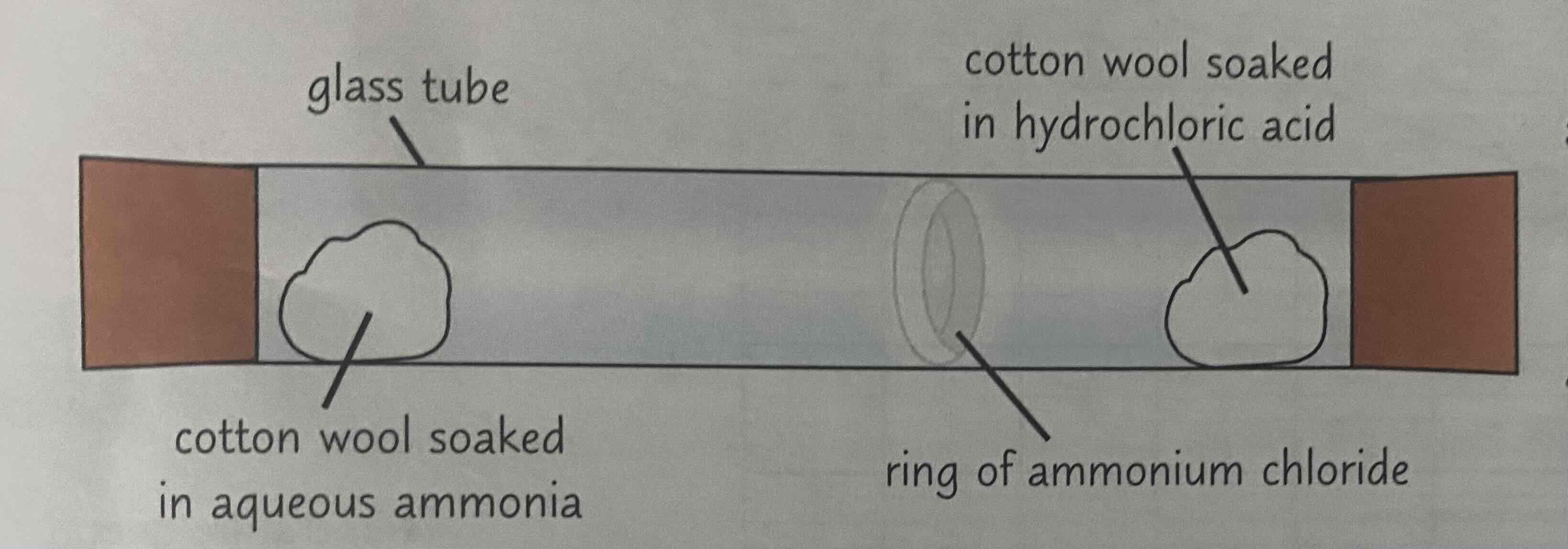
Ammonia and Hydrogen Chloride
Aqueous ammonia gives off ammonia gas. Hydrochloric acid gives off hydrogen chloride gas.
Therefore, you soak one cotton wool with aqueous ammonia and pop it on one end of a glass tube (put a bung on the end too, to contain the ammonia that is given off). And you soak another cotton wool with hydrochloric acid, and pop that in the other end of the glass tube (again, put a bung on).
The ammonia gas will diffuse to the other end of the tube, in the direction of the HCl cotton wool. The hydrogen chloride gas will diffuse to the other end of the tube, in the direction of the ammonia cotton wool.
This will result in a ring of ammonium chloride near the middle of the tube.
(Note that it’s not the exact middle of the tube, because ammonia particles are lighter so they diffuse faster, meaning the ring will be closer to the HCl side)
Look at the image attached.

Bromine Gas and Air
Bromine gas is brown and smells strong.
So, if you fill half of a gas jar with bromine gas and the other half with air - separate the two with a glass plate.
And then remove the glass plate, you will see brown bromine gas slowly diffusing through the air
Solvent
A liquid in which the solute dissolves into
Solute
The substance being dissolved (in a solvent)
Solution
A mixture of a solute and a solvent that does not separate out
Saturated solution
A solution where the maximum amount of solute has been dissolved; no more solute will dissolve in the solution
Solubility
The measure of how much solute will dissolve in a solvent.
Measured in grams of solute per 100 grams of solvent. For example, if 23 grams of a substance can dissolve in 100 grams of water before the solution become saturated, then that substance has a solubility of 23 g per 100 g of water.
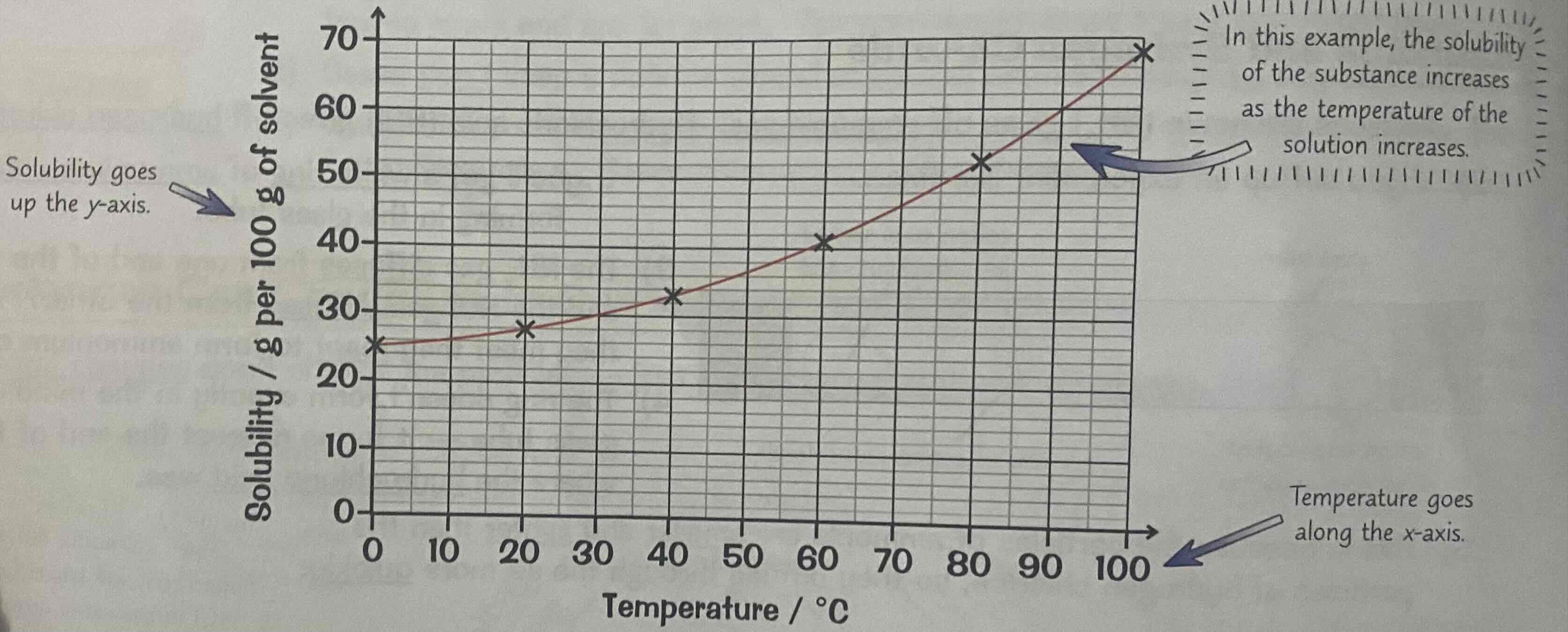
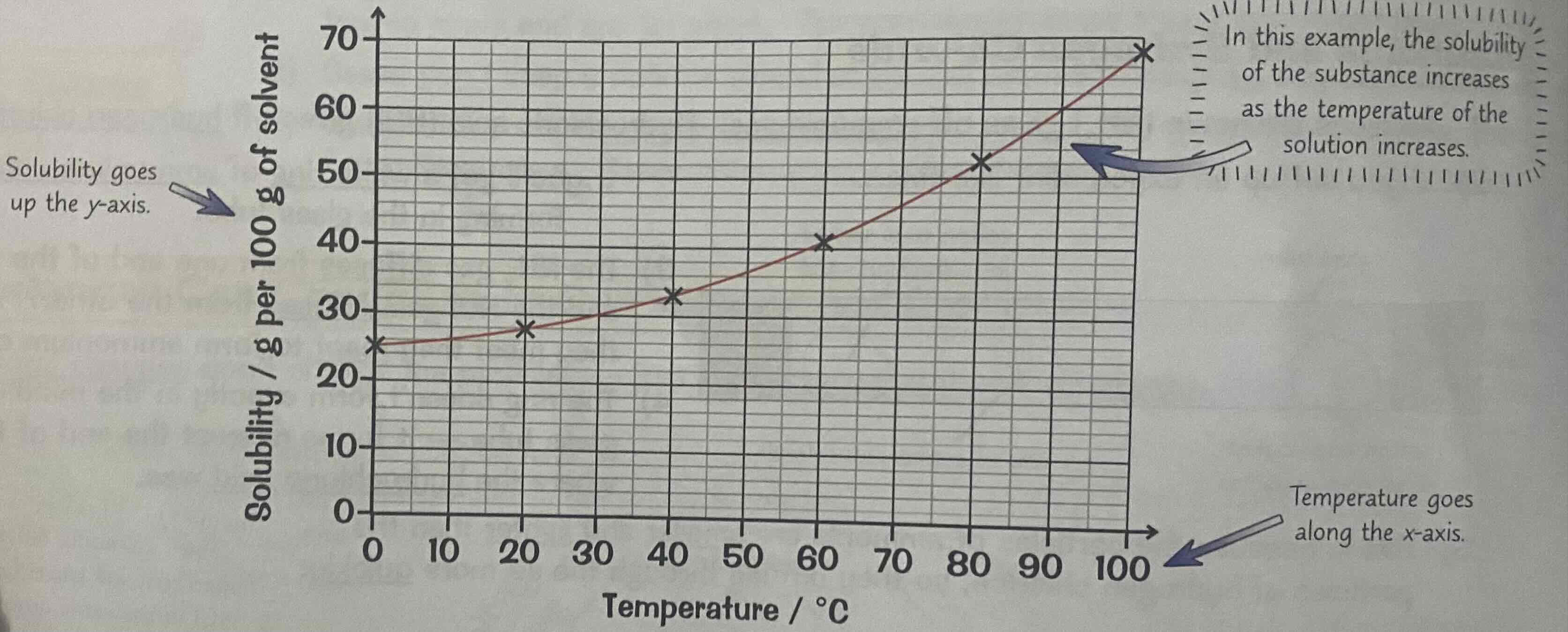
Solubility curves
The solubility of most solid substances increases as you increase the temperature.
A graph of solubility versus temperature is known as the solubility curve.
Solubility goes on the y axis, and temperature goes on the x axis.
So, you can use a solubility curve to see the solubility of a substance at a specific temperature.
Look at the image attached.
Practical: Investigate the solubility of a solid in water at a specific temperature
Let’s use ammonium chloride as our solid for this experiment (obviously you can use other solids).
Make a saturated solution by adding an excess of ammonium chloride into 10cm³ of water in a boiling tube.
Stir the solution and place it in a water bath set to 25°C.
After 5 minutes, check that all of the excess solid has sunk to the tube and use a thermometer to check if the temperature has reached 25°C.
Weight an empty evaporating basin. Pour some of the solution into the basin, making sure not to pour any of the undissolved solid.
Re-weigh the basin and its contents.
Gently heat the basin using a Bunsen burner to remove all of the water.
Once all of the water has evaporated, you are left with pure ammonium chloride. Re-weigh the evaporating basin and its contents.
Repeat steps 1-6 twice more, but with the water at different temperatures (e.g. 35° and 45°C).
You can use all the measurements to calculate the solubility and then you can plot a graph
Reason why your calculated solubility value might be lower than it should be - If you heat the basin in step 6 too strongly, some of the ammonium chloride may turn into a gas and escape. This would cause the mass of the solid in the basin to decrease, therefore you would calculate solubility to be lower than it should be.
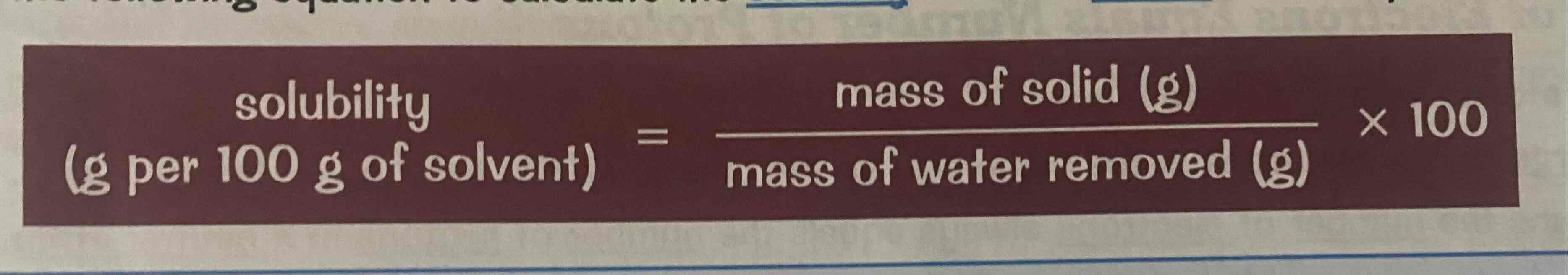
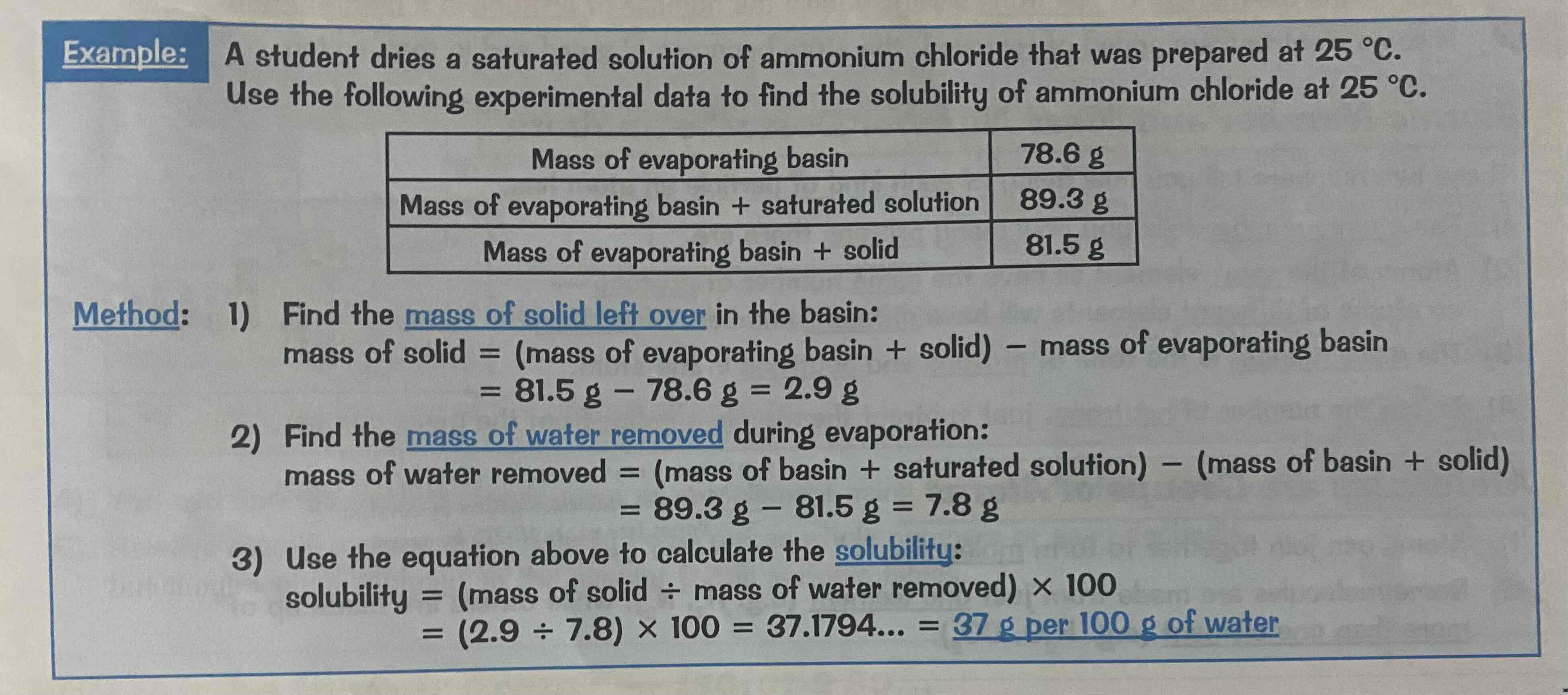
How to calculate solubility
solubility = (mass of solid/mass of water removed) x 100
Look at the image attached.
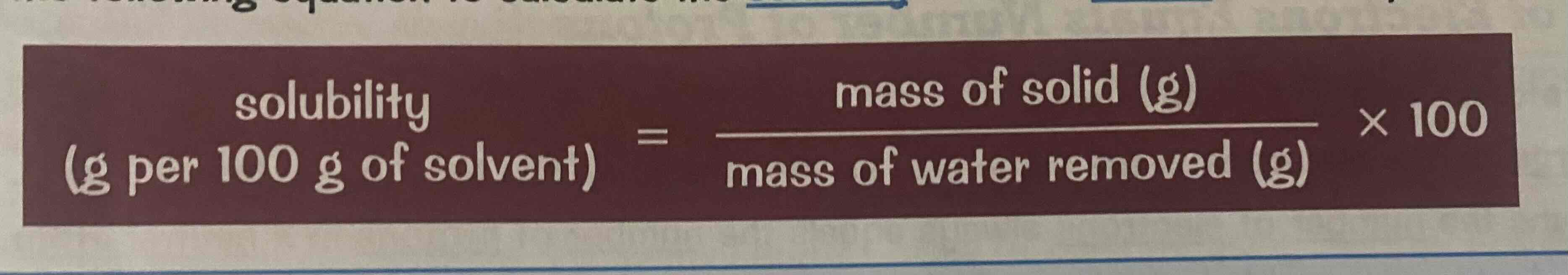
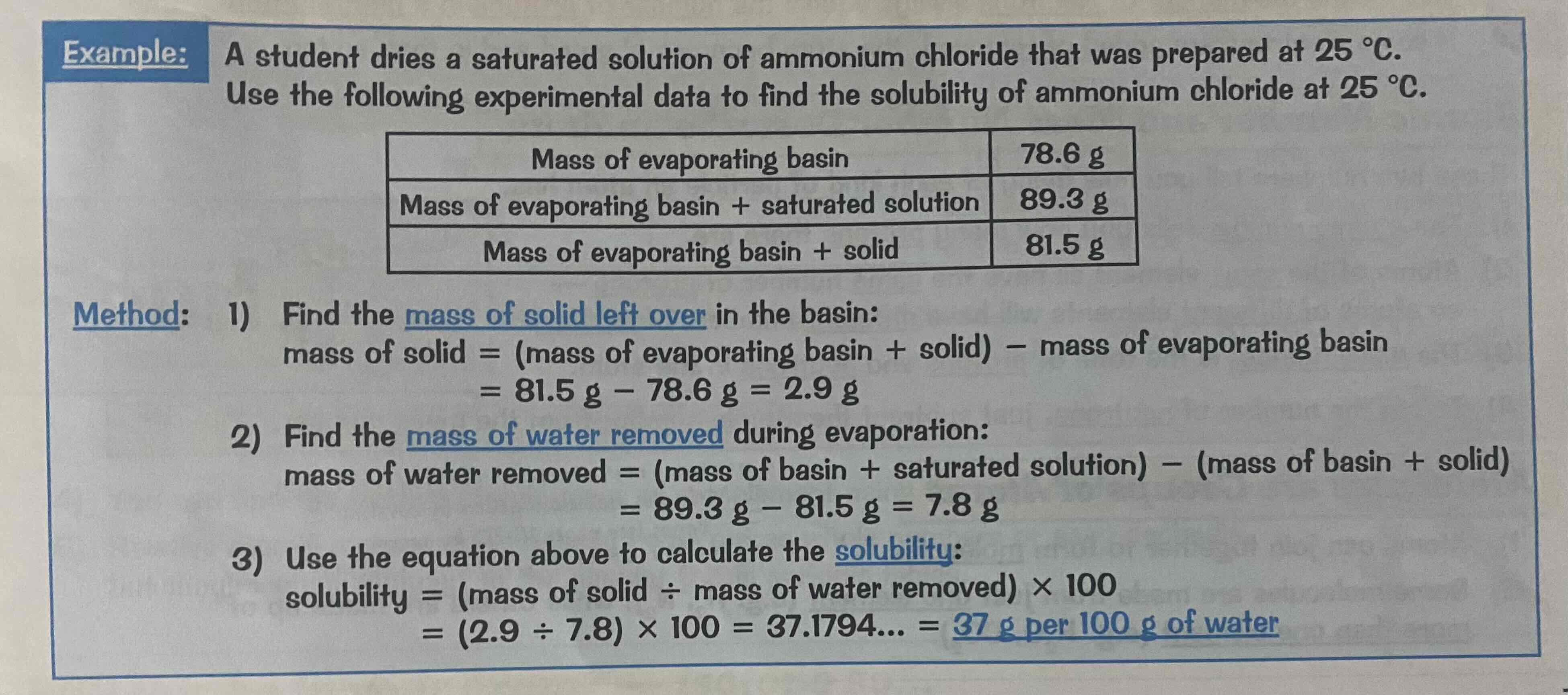
Calculating solubility example question
Look at the image attached.