Inorganic Chemistry Chapter 1
1/11
Earn XP
Description and Tags
"what is inorganic chemistry"
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Quadruply bonded metals contains
delta
Why is the delta bond possible
b/c of the d orbitals interaction
what is a fivefold bond?
A bond involving five pairs of electrons shared between two atoms, typically found in certain transition metal complexes.
What is Bridging hydrogen atoms?
they form bridges across edges or face pf polyhedra of metal atoms
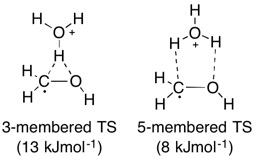
what can also bridge (rarely)
Alkyl groups
How many coordination numbers that the central atoms can have in inorganic compounds?
it can range to 12
what is a ligand?
a molecule or ion that binds to a central metal atom, forming a coordination complex. It can donate electron pairs to the metal and may be neutral or charged.
How is the geometries change in inorganic chemistry?
Both tetrahedral and square planer shape occur for 4 coordinate compound of both metals and nonmetals
coordination complexes
metas are in the center with anions or neutral molecules (ligands) bonded to them (frequency N , O or S)
organometallic complexes
when carbon is the element directly bonded to metal atoms or ions
p4
forms a tetrahedral geometry with no central atom
Inorganic compounds containing pi bonded aromatic rings
metals atoms are sanwhcih between two aromatic rings