Ch 2 ife science
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
Which feature is present in all known living things?
A) genes made from proteins
B) growth from inorganic materials
C) absorption of sunlight for energy
D) metabolic reactions
D) metabolic reactions
Which term best describes the water combined with powder to make lemonade?
A) product
B) reactant
C) solute
D) solvent
D) solvent
Which term best describes the powder combined with water to make lemonade?
A) product
B) reactant
C) solute
D) solvent
C) solute
Which chemical condition describes the electrons in a water molecule being shared unequally between the hydrogen and oxygen atoms?
A) hydrophobic
B) ionic
C) noncovalent
D) polar
D) polar
Which particles are found in the nucleus of an atom?
A) electrons and neutrons
B) neutrons and protons
C) protons and electrons
D) neutrons, electrons, and protons
B) neutrons and protons
Which characteristic would zombies need to develop to be considered alive?
A) consumption of energy-containing molecules in human flesh
B) ability to perform homeostasis and heal wounds
C) production of more zombies through bites or scratches
D) response to external stimuli such as loud noises
B) ability to perform homeostasis and heal wounds
What type of chemical bond connects the complementary strands of a DNA molecule to each other?
A) hydrogen bonds
B) ionic bonds
C) nonpolar covalent bonds
D) polar covalent bonds
A) hydrogen bonds
Which element is the basis for the macromolecules found in living things?
A) carbon
B) hydrogen
C) nitrogen
D) oxygen
A) carbon
Imagine a newly discovered biological molecule that is mostly hydrophobic in its structure. How would this new molecule be classified?
A) carbohydrate
B) lipid
C) nucleic acid
D) protein
B) lipid
What atoms may form a hydrogen bond?
A) two hydrogen atoms
B) two oxygen atoms
C) one hydrogen atom and one partially positive atom
D) one hydrogen atom and one partially negative atom
D) one hydrogen atom and one partially negative atom
What kind of bond is found between the individual atoms of a single water molecule?
A) hydrogen bonds
B) ionic bonds
C) covalent bonds that are not polar
D) covalent bonds that are polar
D) covalent bonds that are polar
What kind of bond holds two water molecules to each other?
A) hydrogen bonds
B) ionic bonds
C) nonpolar covalent bonds
D) polar covalent bonds
A) hydrogen bonds
Which component of amino acids accounts for the differences in their properties?
A) the amino group
B) the carboxyl group
C) the side group
D) the type of bonds
C) the side group
What molecule is composed only of chains and rings of hydrogen and carbon?
A) carbohydrate
B) hydrocarbon
C) polypeptide
D) polysaccharide
B) hydrocarbon
What molecule is composed of one or more sugars?
A) carbohydrate
B) lipid
C) nucleic acid
D) polypeptide
A) carbohydrate
Which monomer units combine to form polysaccharides?
A) amino acids
B) fatty acids
C) nucleotides
D) sugars
D) sugars
Which monomer units combine to form proteins?
A) amino acids
B) fatty acids
C) nucleotides
D) sugars
A) amino acids
Which monomer units combine to form nucleic acids?
A) amino acids
B) fatty acids
C) nucleotides
D) sugars
C) nucleotides
Which macromolecule is a lipid?
A) cellulose
B) cholesterol
C) sucrose
D) ribonucleic acid
B) cholesterol
What is the ability of living things to maintain a relatively constant internal environment?
A) cellular respiration
B) homeostasis
C) metabolism
D) stimulus-response
B) homeostasis
Sodium chloride is composed of molecules that are stable when dry. In water, the atoms of the molecules separate from each other. What type of chemical bond holds the dry substance together?
A) hydrogen bonds
B) ionic bonds
C) nonpolar covalent bonds
D) polar covalent bonds
B) ionic bonds
Which type of macromolecule is present in enzymes?
A) carbohydrates
B) lipids
C) nucleic acids
D) proteins
D) proteins
Which activity is part of a living organism's metabolism?
A) secretion of wastes
B) growth and development
C) responses to external stimuli
D) reproduction
A) secretion of wastes
Which example is an element?
A) water
B) methane
C) calcium
D) carbon dioxide
C) calcium
What type of electric charge do protons carry?
A) negative
B) positive
C) neutral
D) no charge
B) positive
Which example is nonpolar?
A) a positive ion
B) a negative ion
C) a neutral ion
D) a molecule with no partial charges
D) a molecule with no partial charges
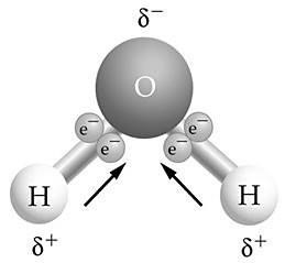
Use the figure to answer the following question. If two or more of these molecules are close to each other, how will they bond together?
A) hydrogen bonding, with two hydrogen atoms bonded together
B) covalent bonding, with two oxygen atoms bonded together
C) hydrogen bonding, with a hydrogen atom bonded to an oxygen atom
D) ionic bonding, with a hydrogen ion bonded to an oxygen atom
C) hydrogen bonding, with a hydrogen atom bonded to an oxygen atom
What kind of molecule forms the bilayer found in cellular membranes?
A) carbohydrate
B) cholesterol
C) phospholipid
D) protein
C) phospholipid
Which feature is found in both prokaryotic and eukaryotic cells?
A) mitochondrion
B) Golgi apparatus
C) DNA
D) chloroplast
C) DNA
Which feature is found in prokaryotic cells?
A) nucleus
B) organelle
C) nuclear membrane
D) cell wall
D) cell wall
How does the diameter of a prokaryotic cell compare with the diameter of a eukaryotic cell?
A) A prokaryote has twice the diameter of a eukaryote.
B) A prokaryote has one-half the diameter of a eukaryote.
C) A prokaryote has one-tenth the diameter of a eukaryote.
A prokaryote has ten times the diameter of a eukaryote
C) a prokaryote has one-tenth the diameter of a eukaryote
What does "hydrophobic" mean?
A) made of water
B) repelled by water
C) attracted to water
D) dissolved in water
B) repelled by water
Which type of lipid includes sex hormones such as testosterone and estrogen?
A) fats
B) phospholipids
C) proteins
D) steroids
D) steroids
How many bonds can carbon form with other elements?
A) 2
B) 4
C) 6
D) 8
B) 4
Why is carbon often involved in chemical bonding?
A) It can form hydrogen bonds with water.
B) It can dissolve other elements.
C) It can form bonds with four other elements.
D) It can form ionic bonds with other elements.
C) it can form bonds with 4 other elements