electrons and ionisation energy
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
what are shells made up of?
atomic orbitals - region around the nucleus that holds up 2e- with opposite spins
types of atomic orbitals
s, p, d, f
s-orbital
electron cloud of a sphere
can hold up to 2 e-
greater the shell number, greater radius of s-orbital
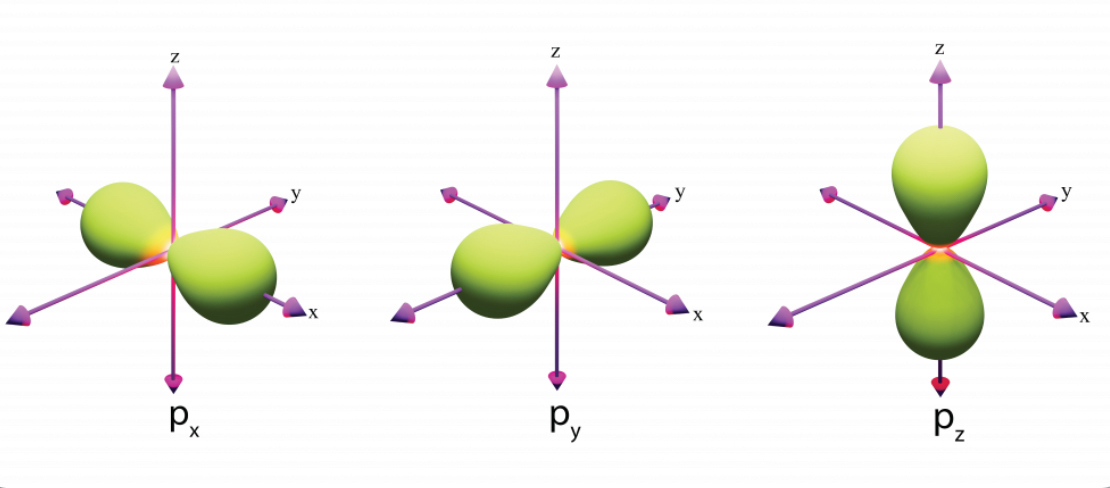
p-orbitals
electron cloud shape of dumb-bell
one orbital can hold up 2e-
the subshells (px, py, pz) hold 6e-
greater shell no. the further the p-orbital is from the nucleus
aufbau principle
electrons fill the lowest energy orbitals first before moving to higher ones
pauli exclusion principle
an orbital can hold a maximum of 2e- and must have opposite spins
hunds rule
electrons fill orbitals of equal energy singly first before pairing up
what are the three rules of electrons?
electrons fills the lowest energy orbital first
an orbital holds up to 2 e- with opposite spins
electrons fills orbitals of equal energy singly first before pairing up
how many electrons can each orbital hold
s → 2
p → 6
d → 10
f → 14
ionisation energy
how easily an e- loses to form positive ions
first ionisation energy
the energy required to remove one e- from each atom in one mole of a gaseous atom of an element to form one mole of gaseous 1+ ions
eg Na(g) → Na+(g) + e-
units of ionisation energy
kJ mol⁻¹
What are the main factors affecting ionisation energy?
Nuclear charge (number of protons)
Distance of outer electron from nucleus
Electron shielding
Electron–electron repulsion (sub-shell effects)
What is meant by successive ionisation energies?
The energies required to remove each electron in turn from the same atom.
Why is the 2nd ionisation energy always higher than the 1st?
Because the electron is removed from a positive ion, so there’s greater attraction between the nucleus and remaining electrons.
What happens to ionisation energy across a period (e.g. Na → Ar)?
increases overall
Why does ionisation energy increase across a period?
Nuclear charge increases (more protons)
Same shielding
Electrons closer to nucleus (atomic radius decreases)
→ Stronger attraction → more energy needed to remove an electron.
What are the exceptions to the trend in ionisation energy across Period 2?
Boron (B) has lower IE than Be → electron removed from 2p, higher energy sub-shell.
Oxygen (O) has lower IE than N → due to paired electrons in 2p orbital causing extra repulsion.
What causes a drop in ionisation energy between Be → B and N → O?
Be → B: change from 2s → 2p, higher energy, easier to remove.
N → O: electron pairing in p orbital increases repulsion.
What happens to ionisation energy down a group?
decreases
Why does ionisation energy decrease down a group?
More electron shells → increased shielding
Outer electrons further from nucleus
Attraction weaker, despite increased nuclear charge.
Which factor has the greatest influence down a group?
Increased distance and shielding outweigh increased nuclear charge.
What does a large jump in successive ionisation energies show?
A change of shell — the electron is now being removed from a closer, more strongly attracted shell.
How can successive ionisation energies identify an element?
The pattern shows how many electrons are in the outer shell (e.g., a big jump after 3 electrons → element is in Group 3).