CMS I Cummulative: Oncology
1/249
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
250 Terms
What tumor marker(s) are associated with breast cancer?
CA 15-3
CA 27-29
CEA
What tumor marker(s) are associated with ovarian cancer?
Ca-125
What tumor marker(s) are associated with testicular cancer?
Alpha fetoprotein (AFP)
beta-hCG
LDH
What tumor marker(s) are associated with prostate cancer?
PSA
What tumor marker(s) are associated with lung cancer?
CEA
What tumor marker(s) are associated with stomach cancer?
CEA
CA 19-9
What tumor marker(s) are associated with pancreatic cancer?
CA 19-9
What tumor marker(s) are associated with hepatocellular cancer?
Alpha fetoprotein (AFP)
What tumor marker(s) are associated with medullary thyroid cancer?
Calcitonin
What tumor marker(s) are associated with neuroendocrine tumors?
Chromogranin
What is a post op marker of thyroid cancer but not medullary?
Thyroglobulin
Colonoscopy screening guidlines for colorectal cancer
- Start at 45
- Complete every 10 years
- Continue until age 75
Colonoscopy screening guidlines for hereditary nonpolyposis colorectal cancer
- Start screening 10 years prior to earliest onset of colorectal cancer in a family member
- Repeat every 1-2 years
What must any pt who presents with rectal bleeding be referred for?
Colonoscopy
What testing is done to evaluate for colorectal cancer - familial adenomatous polyposis?
- Colonoscopy
- Genetic testing
Breast cancer screening for average risk pts
- May start mammograms between 40-44 if desired
- Yearly between 45-54
- Every 2 years for >55 years
- Screening should continue as long as a woman is in good health and expected to live 10 more years or longer
Breast cancer screening for high risk pts
Yearly MRI and mammogram if the following criteria are met:
- Lifetime risk of breast cancer 20% or greater
- Known BRCA1 or BRCA2 gene mutation
- First-degree relative with BRCA1 or BRCA2 mutation and have not had genetic testing done
- Radiation therapy to chest between 10-30 y/o
- Have Li-Fraumeni syndrome, Cowden syndrome, or Bannayan-Riley-Ruvalcaba syndrome, or first degree relatives with one of these syndromes
Prostate cancer screening guidelines
PSA and DRE
- Age 54-69 with average risk → every 2 years or more interval screening
- Age 40-54 for African Americans, +FHx of prostate cancer in a first degree relative diagnosed <65 y/o
Who is prostate cancer screening not recommended in?
>70 y/o or any man with less than 10-15 year life expectancy
What is the hallmark sign of leukemia?
Pancytopenia or leukocytosis with circulating blasts
The presence of blasts in a peripheral blood differential or smear requires what?
Emergent hematology consult!
What is used to definitively diagnose leukemia?
Bone marrow aspiration and biopsy
What is the most common acute leukemia in adults?
Acute myeloid leukemia
Clinical presentation of AML
•Fatigue
•Shortness of breath
•Increased Infection
•Increased bruising/bleeding → epistaxis, gingival bleeding, menorrhagia
•Fever → tumor Fever vs Infection
•Night Sweats
•Bone and joint pain
PE findings for AML
•Pallor
•Petechiae/purpura
•Stomatitis
•Gingival Hyperplasia
•Myeloid Sarcoma
•Leukemia Cutis
•+/- lymphadenopathy
•+/- hepatosplenomegaly
CBC findings with AML
•Anemia
•Neutropenia
•Thrombocytopenia
•Blasts on differential
What would a peripheral smear show with AML?
Auer rods - rod shaped structures in cytoplasm
Bone marrow biopsy findings with AML
>20% myeloblasts
What causes CML?
Overproduction of myeloid cells (primarily neutrophils)
What is unique about CML?
•Characterized by a specific genetic abnormality known as the Philadelphia Chromosome (Ph)
•Philadelphia Chromosome – balanced gene translocation between the long arms of chromosome 9 and 22; i.e. t(9;22)
Clinical presentation of CML
•Often asymptomatic - incidentally discovered on routine CBCs
•Fevers, fatigue
•Splenomegaly
CBC findings with CML
Leukocytosis with predominance of neutrophils
What is special about Imatinib (Gleevec)?
First-in-class BCR-ABL tyrosine kinase inhibitor
- Oral medication taken indefinitely with >90% control of disease
- 100% response rate. Well tolerated drug with little side effects.
What is Imatinib used to treat?
CML
What is the most common childhood malignancy?
ALL (affects lymphoblast)
S/S of acute onset of ALL
•Fever
•Fatigue
•Increased infections
•Increased bleeding/ bruising
•Focal neurologic deficits/seizures due to CNS involvement
•Petechiae
•Pallor
•Splenomegaly
•Hepatomegaly
•Lymphadenopathy
(ALL = anemic, lumpy, limping)
CBC findings with ALL
•Marked anemia (=pallor)
•Neutropenia (=increased infections)
•Thrombocytopenia (=petechiae)
•Blasts on differential
•kidney injury
Bone marrow biospy findings with ALL
>20% lymphoblasts
What treatment is given with ALL in both adults and peds?
CNS prophylaxis due to high prevalence of CNS disease
What is the most common leukemia overall?
CLL (neoplastic transformation of the mature B-lymphocyte)
- elderly primarily, >71
Clinical presentation of CLL
•Fatigue
•Lymphadenopathy
•Hepatomegaly/Splenomegaly
•Asymptomatic- Discovered on incidental CBC
(asymptomatic and lumpy)
CBC findings with CLL
•Increase Absolute Lymphocyte Count (ALC)
•Lymphocytosis > 5,000
•+/- anemia, thrombocytopenia
Bone marrow biopsy findings with CLL
Predominance of mature lymphocytes
What would be found on a peripheral smear with CLL?
Smudge cells - fragile B-lymphocyte break as a result of smear creation
(CLL - crushed little lymphocytes)
How is CLL staged?
•Using Rai (more common in US) and Binet (more common in Europe) system
•Utilizes lymphadenopathy, splenomegaly and cytopenias to determine prognosis
Treatment for CLL
•Active Surveillance - “watch and wait”
•Initiate treatment only when disease causes symptoms (chemo or allogenic stem cell transplant)
What conditions is Hodgkin Lymphoma associated with?
EBV and HIV
Clinical presentation of Hodgkin Lymphoma
•Asymptomatic lump
•B-symptoms → Fevers, night sweats, weight loss
•Pruritus
•Pain on drinking alcohol
•Lymphadenopathy – usually cervical initially (non-tender)
•Leukocytosis/eosinophilia
How is Hodgkin Lymphoma evaluated?
•Excisional biopsy (NOT FNA)
•CBC
•ESR/LDH – markers of cell turnover
•PET/CT neck, chest, abdomen, and pelvis
•Bone marrow biopsy if advanced disease
What would pathology show with Hodgkin Lymphoma?
Reed-Sternberg Cell (not found in non-Hodgkin)
•Large, bi-nucleate cell
•“Owl’s eye” appearance
•Surrounded by healthy reactive cells
•Presentation in a single node initially, typically spreads in contiguous pattern
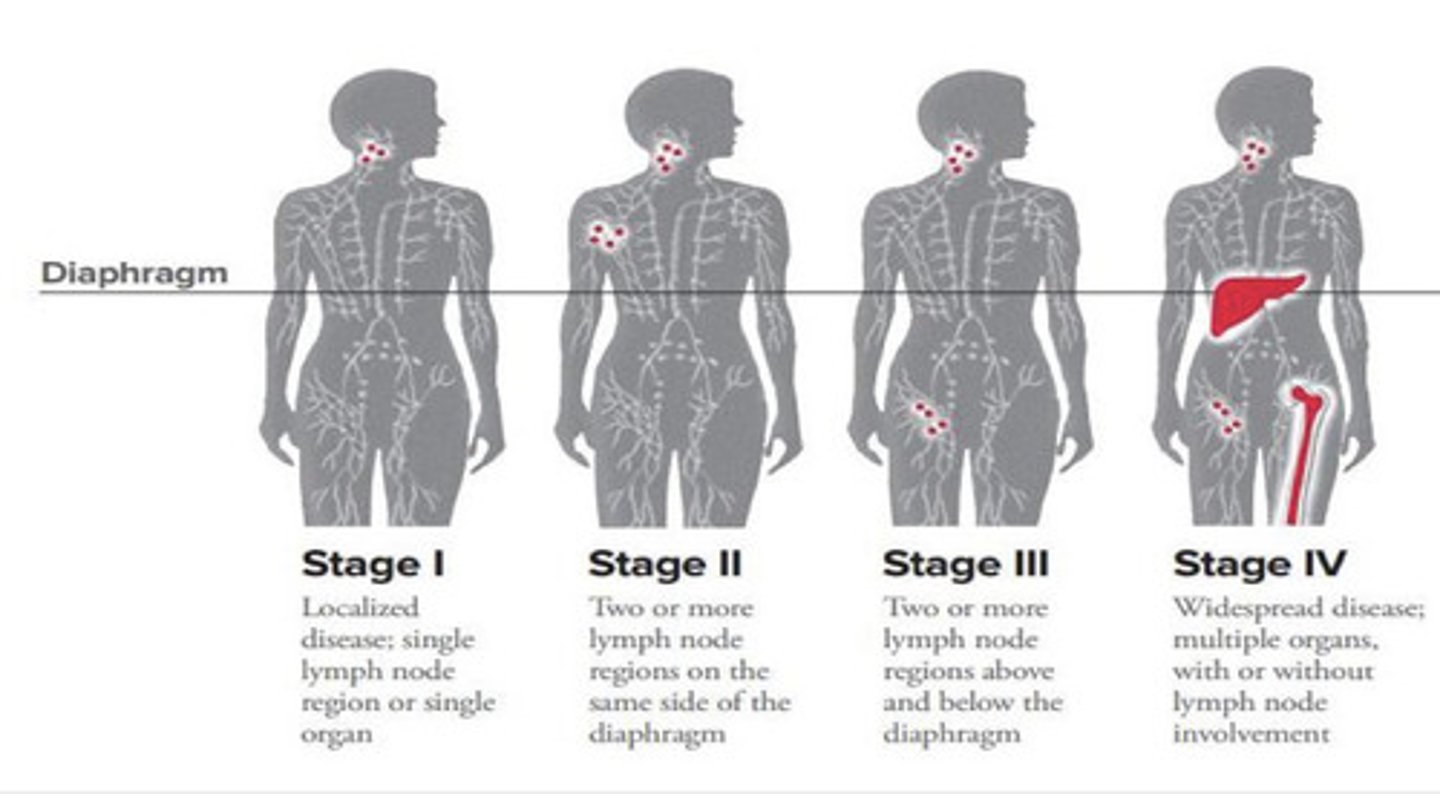
How is Hodgkins Lymphoma staged?
Modified Ann Arbor Staging
Chemotherapy regimen for Hodgkins Lymphoma
ABVD x 6 cycles
- Adriamycin (Doxorubicin) – need echo or MUGA scan 1st before use causes cardiotoxicity
- Bleomycin – pulmonary toxicity or pulmonary fibrosis → need PFT prior to starting [Brentuximab now replacing Bleomycin (less side effects and equal or slightly increased response rates)]
- Vinblastine
- Dacarbazine
How do you evaluate non-Hodgkin lymphoma?
•Lymph node biopsy –core biopsy acceptable, but excisional always preferred
•CT neck, chest, abdomen, pelvis or PET/CT
What is the most common non-Hodgkin lymphoma?
Diffuse large b-cell lymphoma (DLBCL)
Pathognomic finding with Burkitt Lymphoma
Diffuse infiltration of small non-cleaved lymphocytes mixed with large cells – “starry sky” appearance
How many women will develop breast cancer during their lifetime?
1 in 8
Risk factors for breast cancer
•Personal history of breast disease
•+ FHx breast or ovarian cancer
•Dense breasts
•Age > 55 y/o
•Menarche < 12 y/o
•Menopause > 55 y/o
•Age @ first birth > 30 y/o or nulliparous
•Obesity/physical
inactivity
•Alcohol intake > 2-5 drinks daily
•HRT or OCP use (estrogen with +/- progestin)
•History of radiation exposure to chest
•DES (synthetic estrogen) exposure
What may be appropriate if there is a family history of breast cancer?
Surveillance & more intensive screening may be appropriate at a younger age
What genetic alterations are present with breast cancer?
- BRCA1
- BRCA2
- Overexpression of HER2
- p53 mutations
BRCA1 and BRCA2 are....
Tumor suppressor genes
What is HER2?
•Oncogene (previously called HER2/neu)
•Results from an increase in gene number, not gene mutation
•Breast cells grow & divide in an uncontrolled manner
Which gene alteration leads to cancers that often recur & are more aggressive?
HER2
PE findings with breast cancer
Most often - single, non-tender, firm, immobile mass → most commonly in the upper outer quadrant
Less often:
•Nipple discharge or retraction
•Breast enlargement or shrinkage
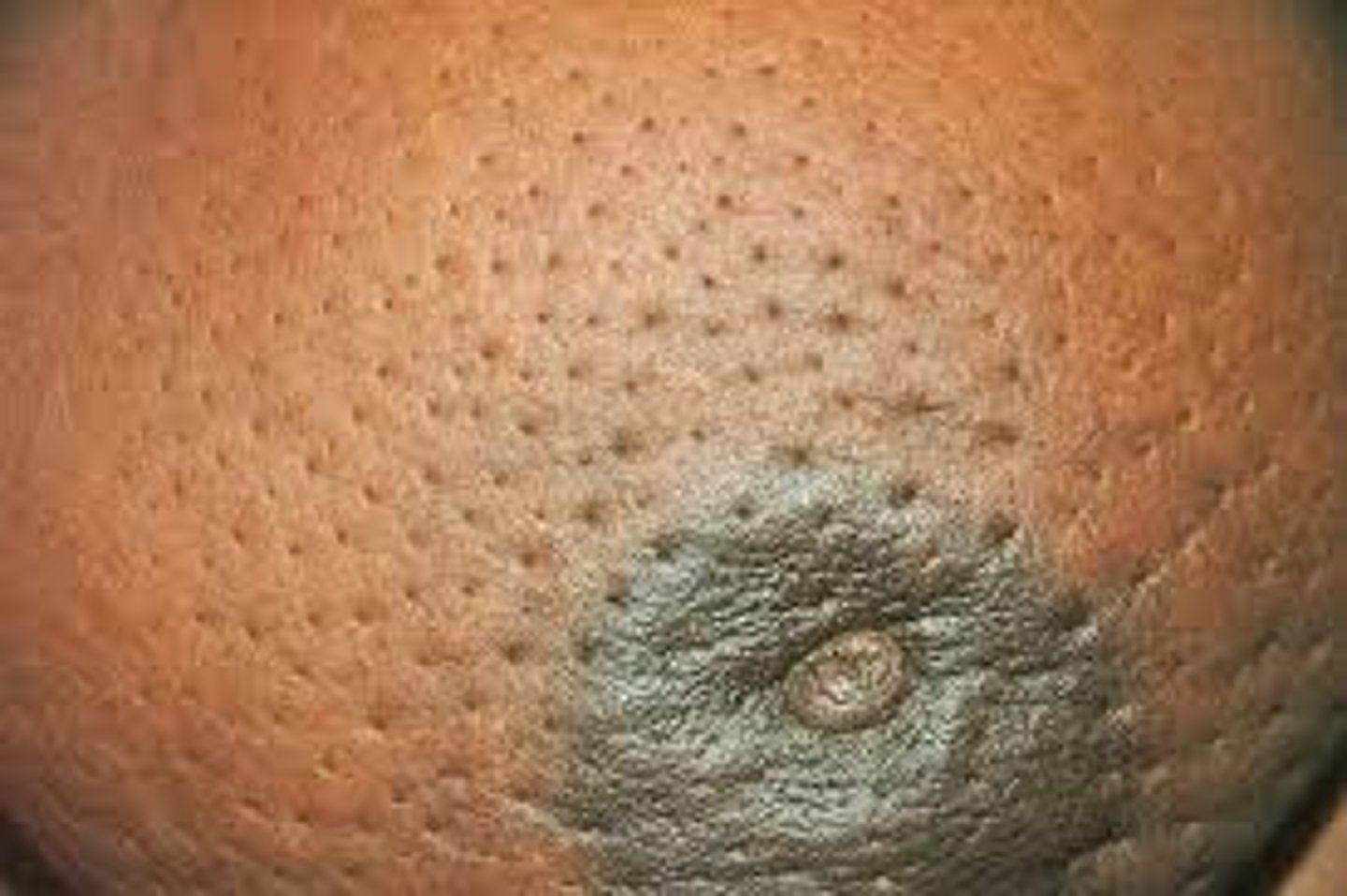
•Skin thickening or changes (peau d’orange)
•Palpable axillary or supraclavicular lymph nodes
If a patient presents with skin thickening or changes (peau d'orange), what would you do next?
Immediate referral
How is breast cancer diagnosed?
•Ultrasound and/or mammogram to confirm suspicious physical exam findings
•Fine needle aspirate (FNA) or stereotactic core-needle biopsy of nodule/mass, excisional biopsy always preferred if possible
If a pt has any positive or suspicious findings on mammogram, what are the next steps?
Surgical oncology referral
What is DCIS?
Ductal carcinoma in situ
•Noninvasive tumor in the breast ducts
•Can progress to invasive cancer
What is the most common type of invasive breast cancer?
Infiltrating breast carcinomas
What is Tamoxifen?
•A selective estrogen receptor modulator (SERM) that inhibits the growth of breast cancer cells by competitive antagonism
•20mg pill that is taken daily
How breast cancer staged?
•Uses TNM system
•Ipsilateral axilla is often staged with sentinel node biopsy to evaluate node involvement
Which breast cancer has the worst survival, higher likelihood of metastatic disease, and limited treatment options?
Triple negative (ER, PR, HER2)
What biomarkers are associated with breast cancer?
•Estrogen-receptor (ER): presence associated with more indolent course; treat with hormone therapy (Tamoxifen)
•Progesterone-receptor (PR)
•HER2: presence allows treatment with trastuzumab (Herceptin)
Treatment for localized breast cancer
Lumpectomy
Treatment for breast cancer - larger tumors or regional spread
Mastectomy
Adjuvant treatment for breast cancer
XRT and/or chemotherapy following surgical resection
•Taxol/Adriamycin/Cyclophosphamide
•Estrogen-Receptor Positive (ER+) - Aromatase inhibitors for 5 years (block production of estrogen)
•Her2/neu Positive (HER2+) -Trastuzumab (Herceptin) therapy, anti-Her2 monoclonal antibody
Why is neo-adjuvant treatment used for breast cancer?
It can reduce tumor burden allowing more successful surgical resection
Treatment for metastatic breast cancer
Chemotherapy and XRT
- Goal of therapy is to control disease and patient symptoms. Not curable.
What is critical following successful treatment of breast cancer? Why?
Recurrence is possible, so close monitoring and follow up with oncologist is critical. Highest in first 5 years after therapy
What breast cancer treatment can be used to treat women before and after menopause?
Selective estrogen receptor modulators (SERM) - raloxifene (Evista), tamoxifen (Soltamox), toremifene (Fareston)
•Block the effect of estrogen in the breast tissue
What breast cancer treatment is the best type of hormonal therapy to start with for postmenopausal women?
Aromatase inhibitor - Exemastane (Aromasin), anastrozole (Arimidex), and letrozole (Femara)
•Stops production of estrogen
•Blocks aromatase enzyme that turns androgen into small amounts of estrogen in the body
What causes multiple myeloma?
Malignant plasma cells over produce antibodies (paraprotein) which is responsible for symptoms of MM
•Plasma cells- most mature form of B-cells. Responsible for antibody production
What is common with multiple myeloma?
Bony disease is common due to plasma cell activation of osteoclasts and inhibition of osteoblasts
Clinical presentation of MM
•Bone pain
•Pathologic fractures – ribs, femoral neck, vertebrae
•Pallor
•Fatigue
•Acute Renal Failure
•Infections – inability to make normal antibodies
•Hyperviscosity syndrome
What causes hyperviscosity syndrome?
•Due to extreme levels of antibodies creating "sludging" of the blood
•Mucosa bleeding, vertigo, nausea, visual disturbances, AMS
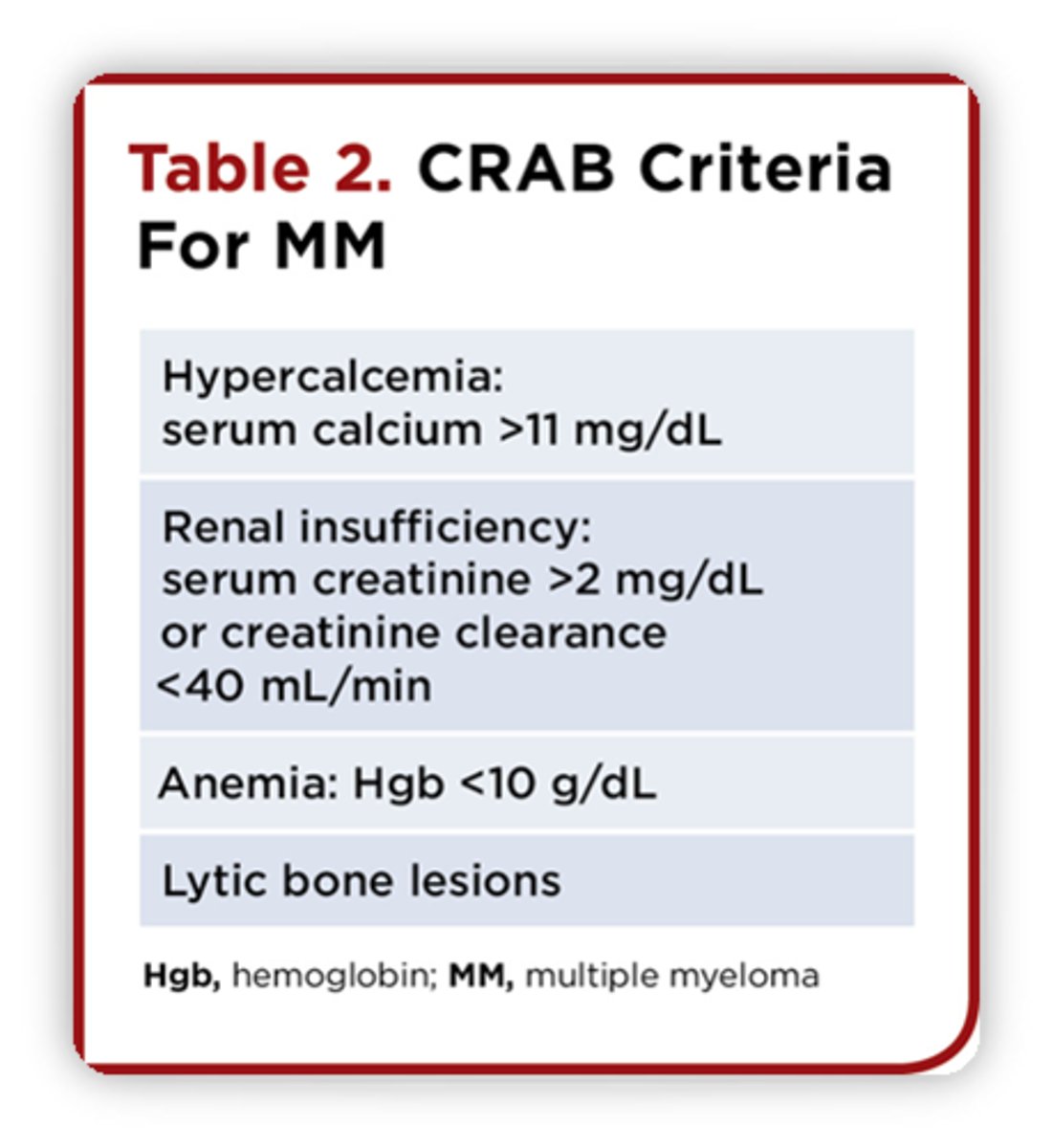
Criteria for diagnosing multiple myeloma
CRAB criteria
What can be seen on peripheral blood smear with multiple myeloma?
Rouleux formation
•RBC's are stacked together in long chains
•Happens with increased serum proteins, particularly fibrinogen and globulins
•Occurs when plasma proteins are high
•Also, increases ESR
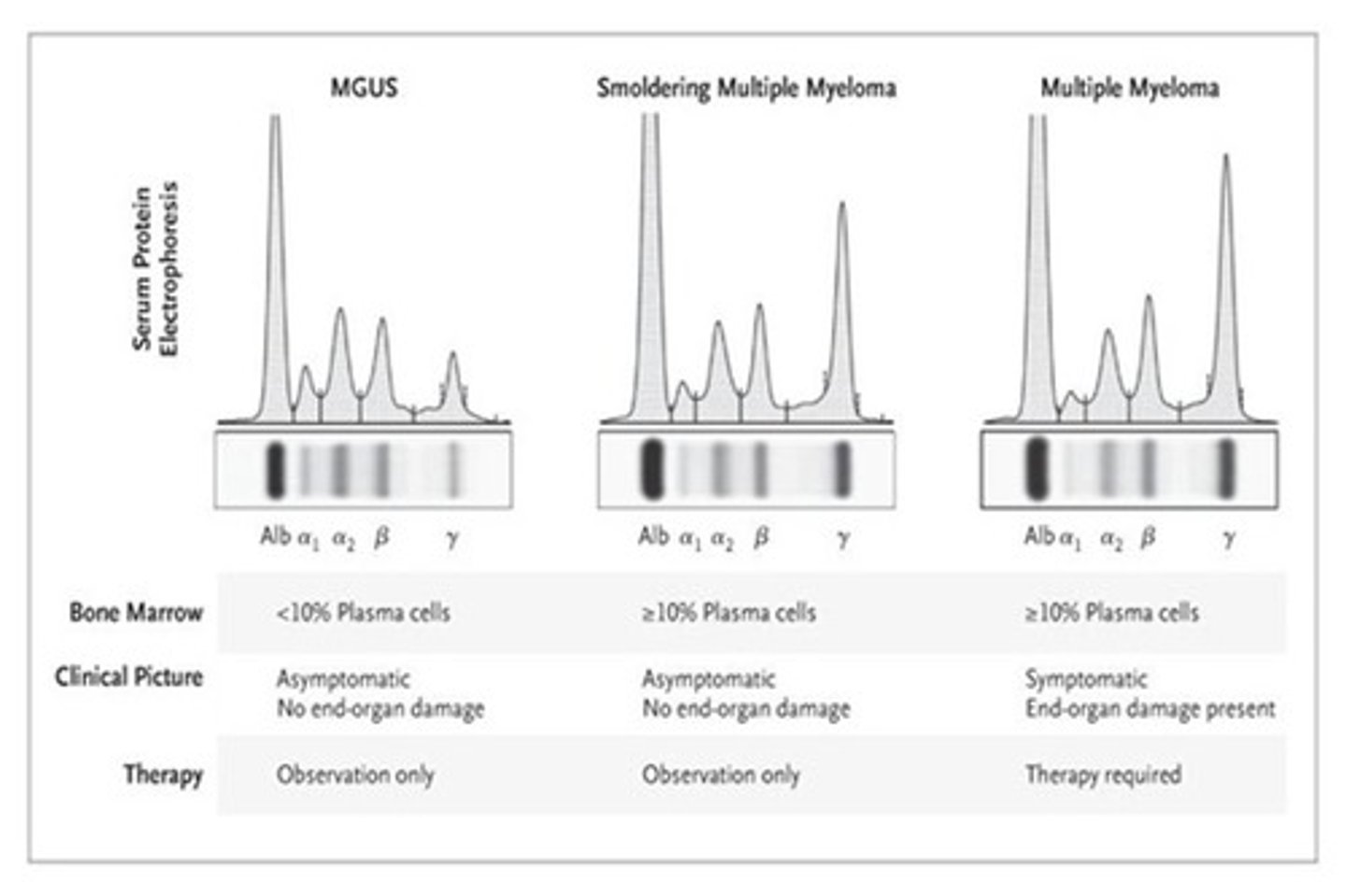
How does MGUS differ from MM?
MGUS:
•Premalignant state of myeloma
•Asymptomatic
•Bone marrow plasma cells <10%, without end-organ damage (CRAB)
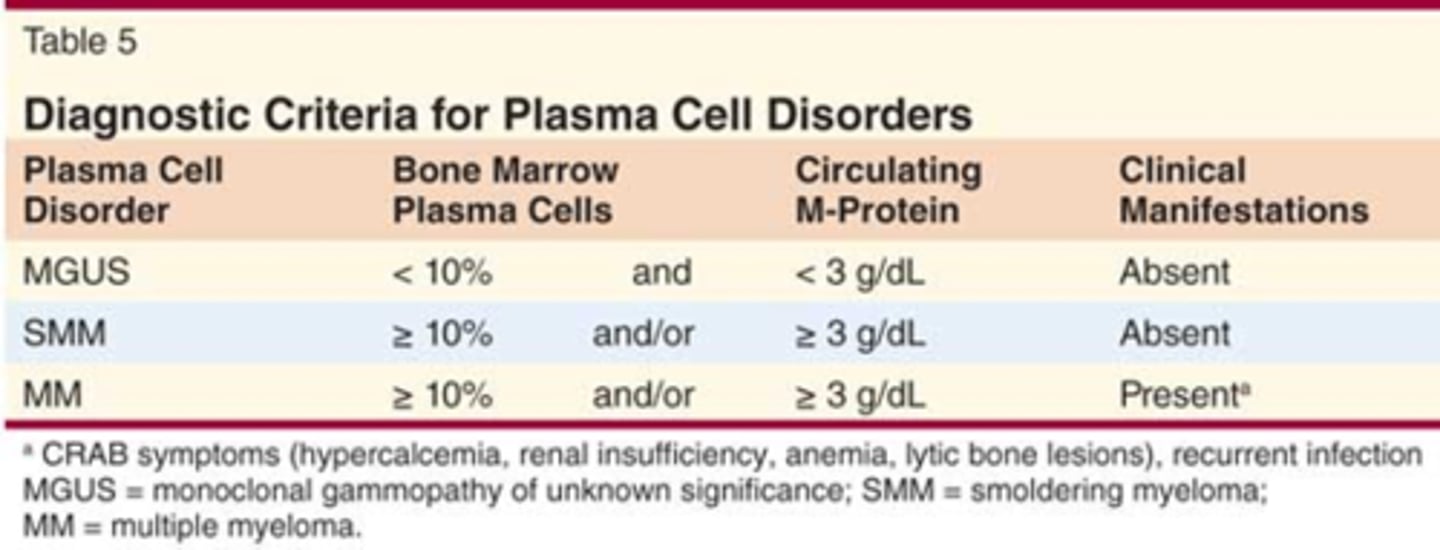
Differences between MGUS and MM
MGUS and MM both have M spike! (according to exam question)
Most common risk factors for cancer
- Tobacco use
- Aging
- UV light
- Ionizing radiation
- Mutated or deleted tumor suppressor genes
- Overexpression or mutation of oncogenes
- Some viruses
- Family history
- Alcohol
- Sedentary lifestyle, poor diet, obesity
- Hormones
What is the most common and preventable cause of cancer?
Tobacco
What is useful for determining both prognosis and treatment?
Molecular testing
Where does the spinal cord end?
L1
What can cause spinal cord compression?
•Cancers that metastasize to the spinal column → lung cancer, breast cancer, multiple myeloma, Hodgkin and non-Hodgkin lymphoma, and prostate cancer
•Most commonly arises in the thoracic spine
•Injury occurs from: edema, hemorrhage, pressure induced ischemia
What happens if there is persistent spinal cord compression?
Can produce irreversible changes to myelin sheaths → neurological impairment
S/S of spinal cord compression
•Back pain at the level of the tumor mass → usually precedes other symptoms by several weeks
•Aggravated by: lying down, usually worse at night; weight bearing; sneezing or coughing
•Progressive weakness
•Sensory changes
•Bowel and bladder dysfunction can progress to incontinence → Often a late sign
•Ataxia
How is spinal cord compression diagnosed?
•MRI with complaints of new onset back pain → emergent with signs of cord compression
•If in doubt, image the entire spine
•Don’t want to miss a tumor!
Treatment for spinal cord compression
•Dexamethasone IV q 4-6h
•Serves to reduce the edema in the spinal cord in effort to spare any remaining viable cord
What is the most common paraneoplastic endocrine syndrome?
Hypercalcemia
Late findings of hypercalcemia
•Muscular weakness
•Hyporeflexia
•Confusion
•Psychosis
•Tremor
•Lethargy
Why does serum calcium need to be corrected?
Total serum calcium concentrations in patients with low or high serum albumin levels may not accurately reflect the physiologically important ionized (or free) calcium concentration (i.e., because albumin is low, free calcium will be higher)