2. PHAR4813 Nanoparticles (127)
1/126
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
127 Terms
Specificity of nanoscale materials (5)
1. atoms and molecules are generally less than 1 nm, and we study them in chemistry
2. condensed matter physics deals with solids with infinite array of bound atoms
3. nanoscience deals with the in-between meso-world
4. surface to volume ratio
5. size-dependent properties
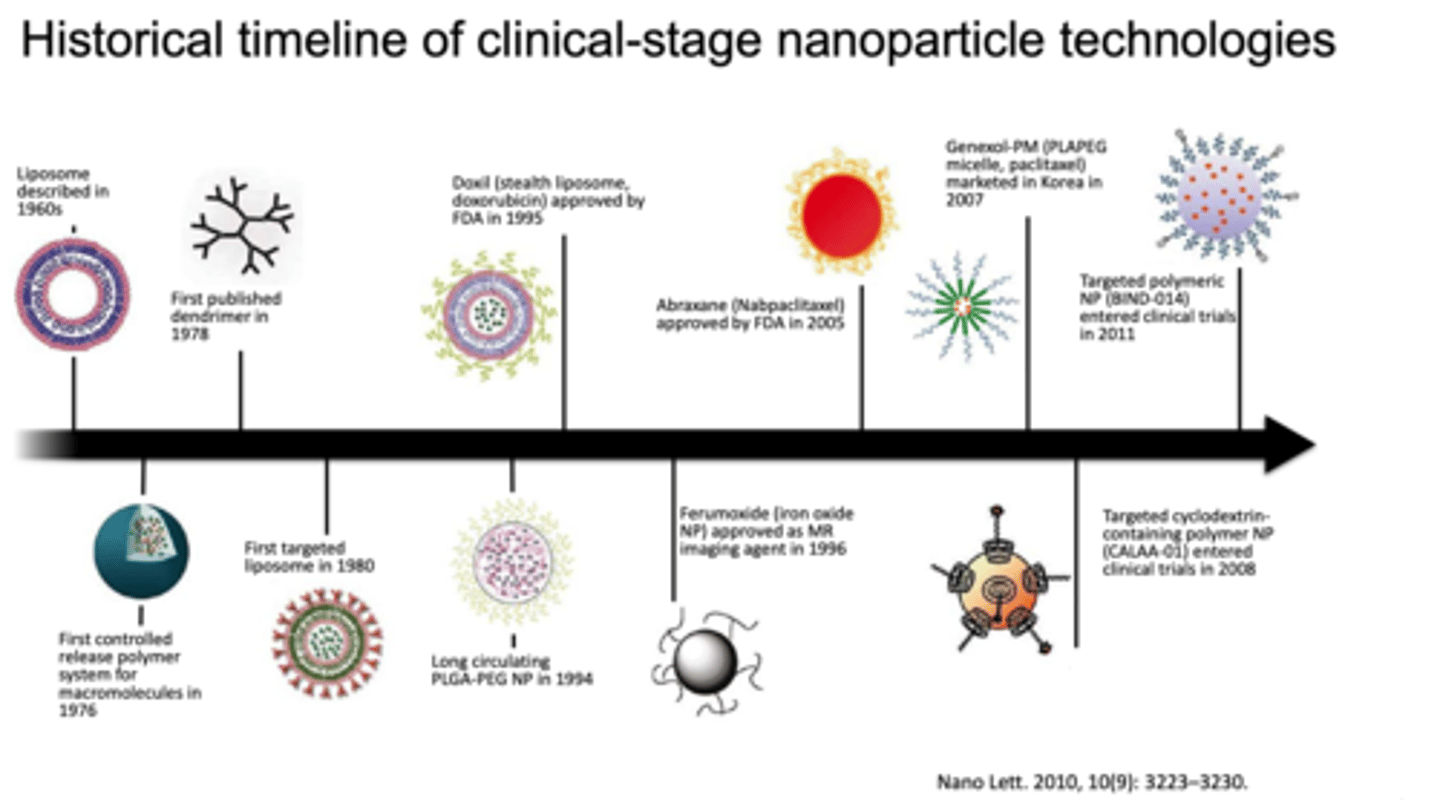
Historical timeline of clinical-stage nanoparticle technologies
see image
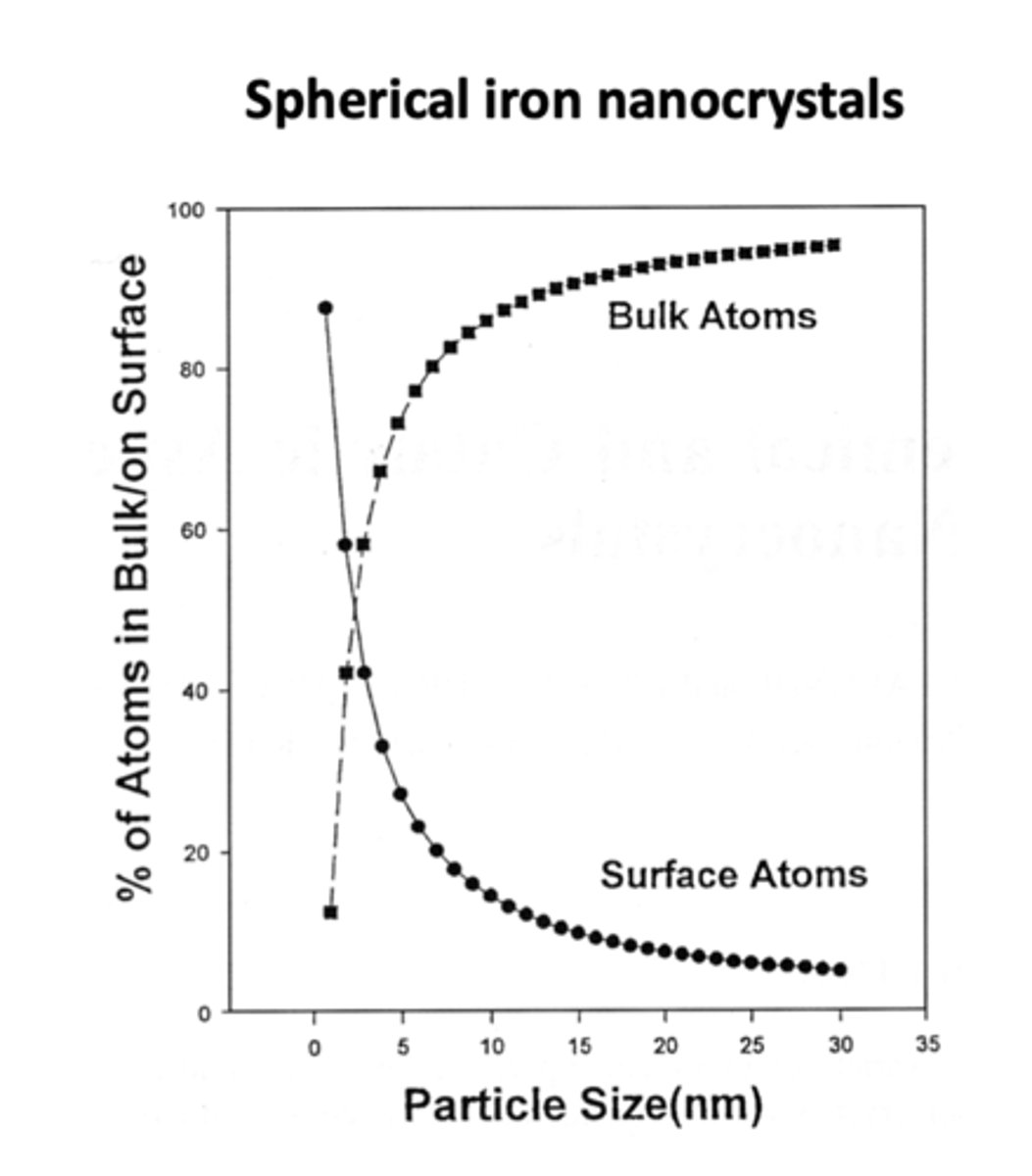
Surface to volume ratio of nanoparticles
a 3 nm iron particle has 50% atoms on surface; a 10 nm particle has 20% on surface; a 30 nm particle has only 5% on surface.
What is a feature of nanoscale?
high ratio of surface area to volume
List 3 examples of nanoparticles
1. Gold nanoparticles
2. Quantum dots (QD)s
3. Liposomes and lipid nanoparticles
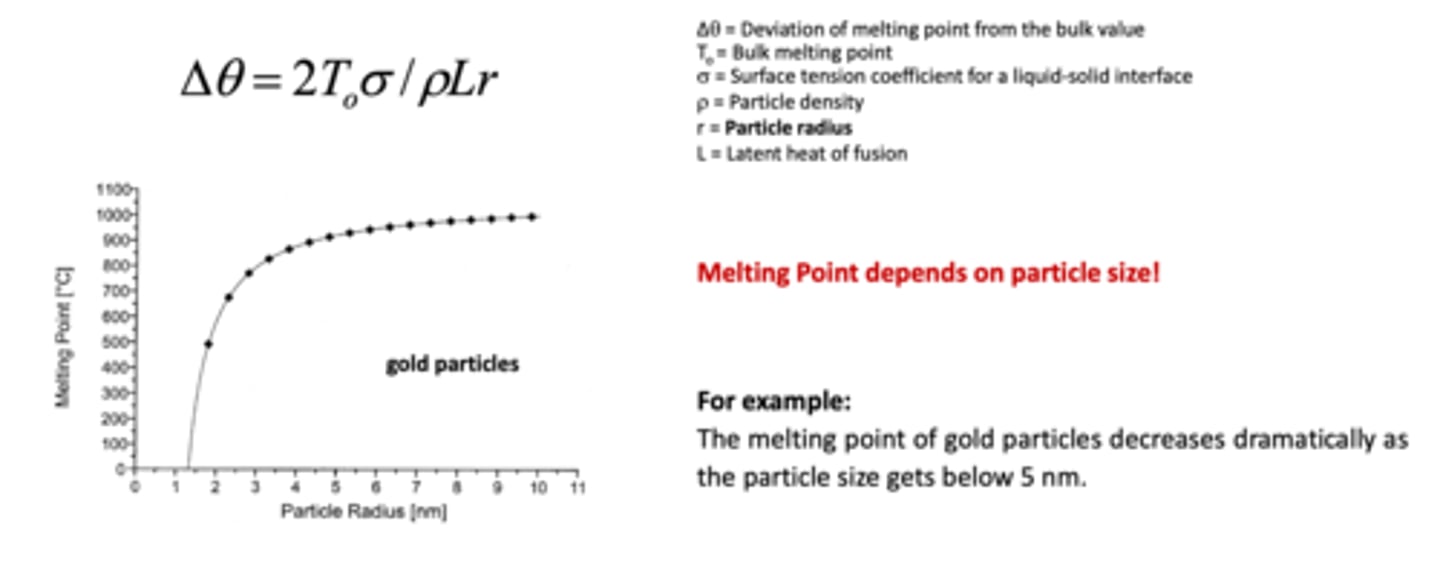
What is the relation between size and melting point of metallic nanoparticles?
melting point depends on particle size
e.g. the melting point of gold decreases dramatically as the particle size gets below 5 nm.
What is surface plasmon resonance? (3)
- occurs when the light's properties changes when light is shone against gold nanoparticle (the metal surface)
- an optical technique used to measure molecular interactions in real time
- the light (strength, intensity, spectrum) changes after each interaction:
A. interaction of gold nanoparticles with light
B. interaction of gold nanoparticles with light after interacting with biological molecules such as DNA and enzymes --> by measuring the light change properties, we can find out interaction between nanoparticles vs biological materials
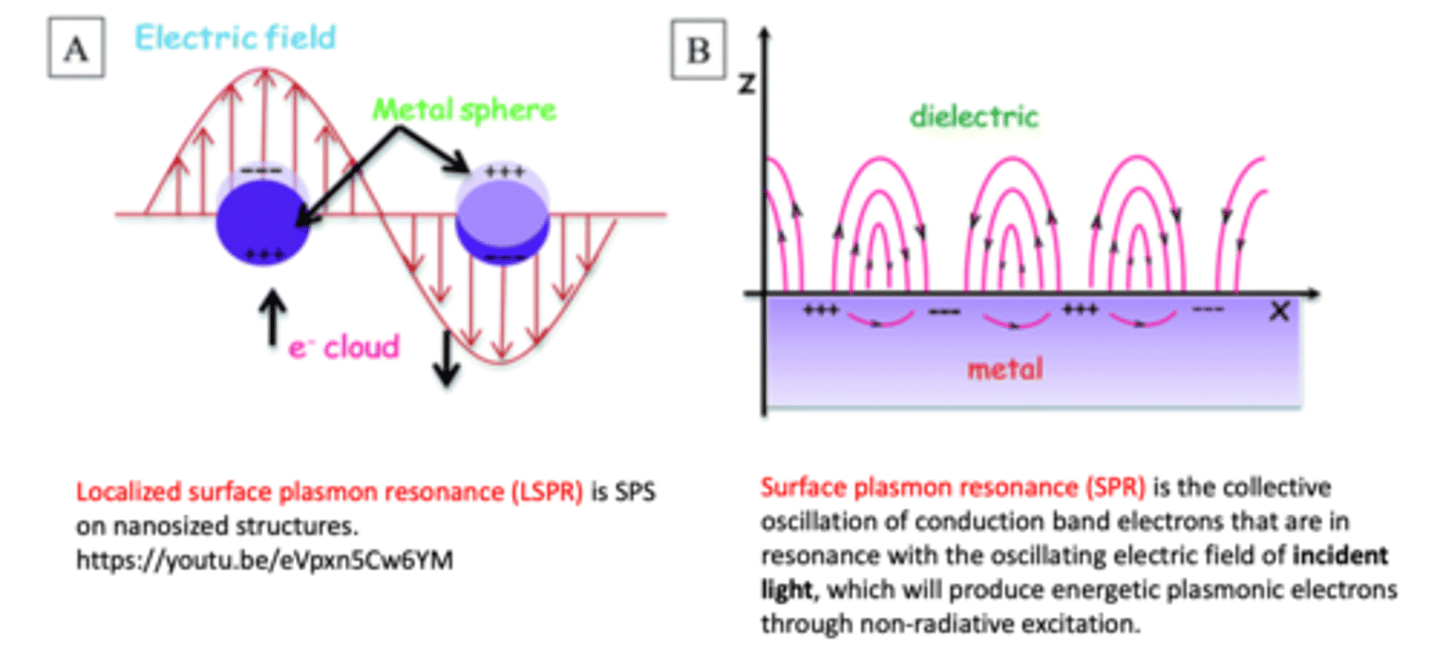
What is localised surface plasmon resonance (LSPR)?
when the resonance is coherent with that of the external optical field, nanoparticles exhibit strong absorption at this wavelength of the external field --> absorption is transferred into heat
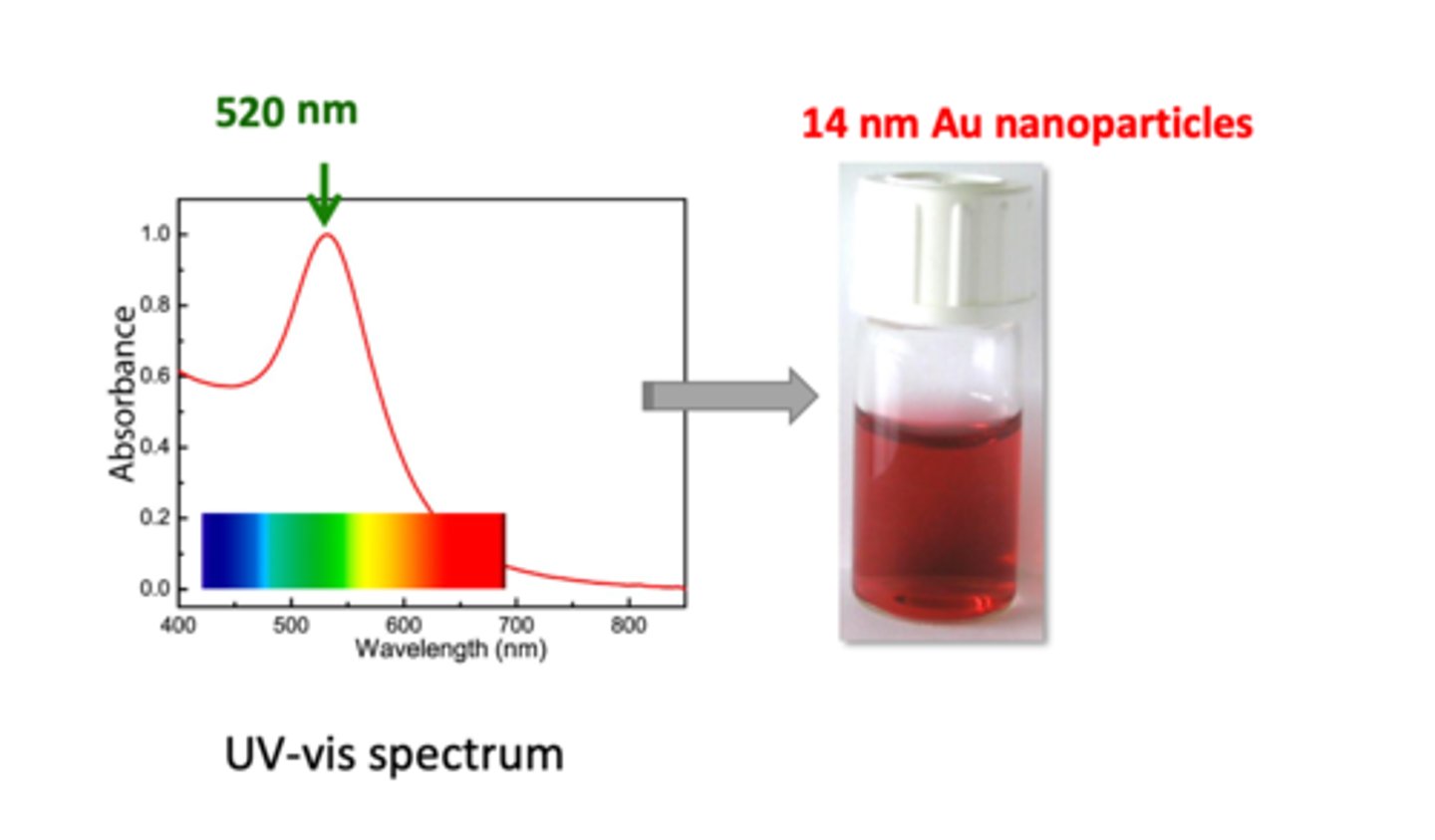
Explain the UV-vis spectrum of gold nanoparticles in the diagram (3)
Below: Y axis shows absorbance, X axis shows wavelength with light visible to the eye:
1. diagram spectrum shows 520 nm being the strongest absorbance for gold nanoparticles
2. when white light is passed through 14 nm Au sample, all of the green photons will be absorbed (520 nm is on the green wavelength) --> what is left is red colour in the solution
3. from the diagram, gold absorbance decreases dramatically after 520 nm (about 0.1 after 650 nm) --> this means all other colours will be absorbed except red --> dispersion looks red in colour
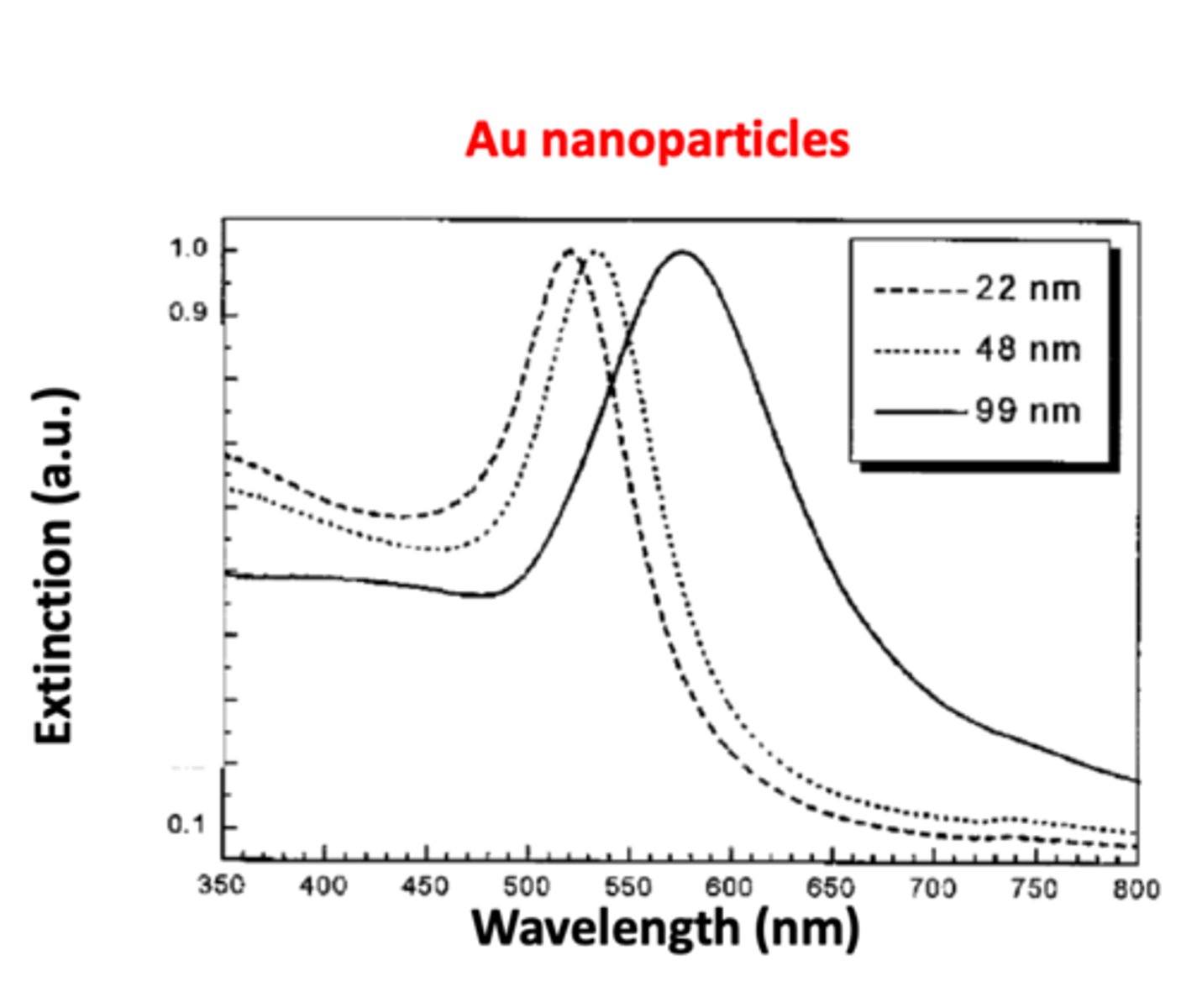
Explain the relationship between nanoparticle size and surface plasmon resonance of metal nanoparticles (3) SEE GRAPH
1. as the diameter of the gold nanoparticle increases, the absorption curve will also shift towards the bigger wavelength --> this will explain the aggregation phenomenon
2. surface plasmon resonance of metal nanoparticles red shifts (shifts towards bigger wavelength) with increasing nanoparticle sizes
3. uneven excitation of free electrons for bigger nanoparticles leads to the "retardation effect" and correspondingly the red shift (move to longer wavelength) of the SPR
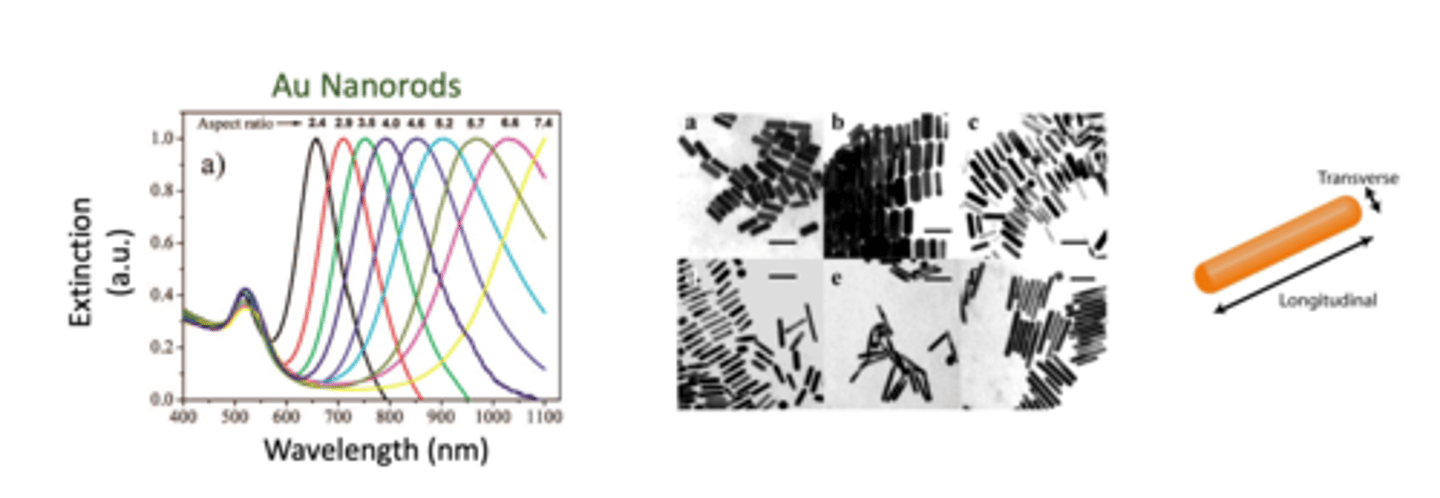
How does the shape of the gold nanoparticle influence wavelength absorption? SEE UV VIS PROFILE (4)
- gold nanoparticle shape will also influence wavelength absorption
- the shape property of the gold nanoparticle is important because it can be characterised using spectrometry profiling
- gold nanoparticle ini rod-shape (nanorods) exhibit both transverse and longitudinal surface plasmon resonances (along with their 2 axes)
- their longitudinal peaks are very sensitive to their aspect ration (L/W ratio) (larger ratio leads to longer wavelength) and can cover a wide spectral range
- as the longitudinal length increases, L/W ratio increases (aspect ratio) --> wavelength shifts towards longer wavelength (red-shifts)
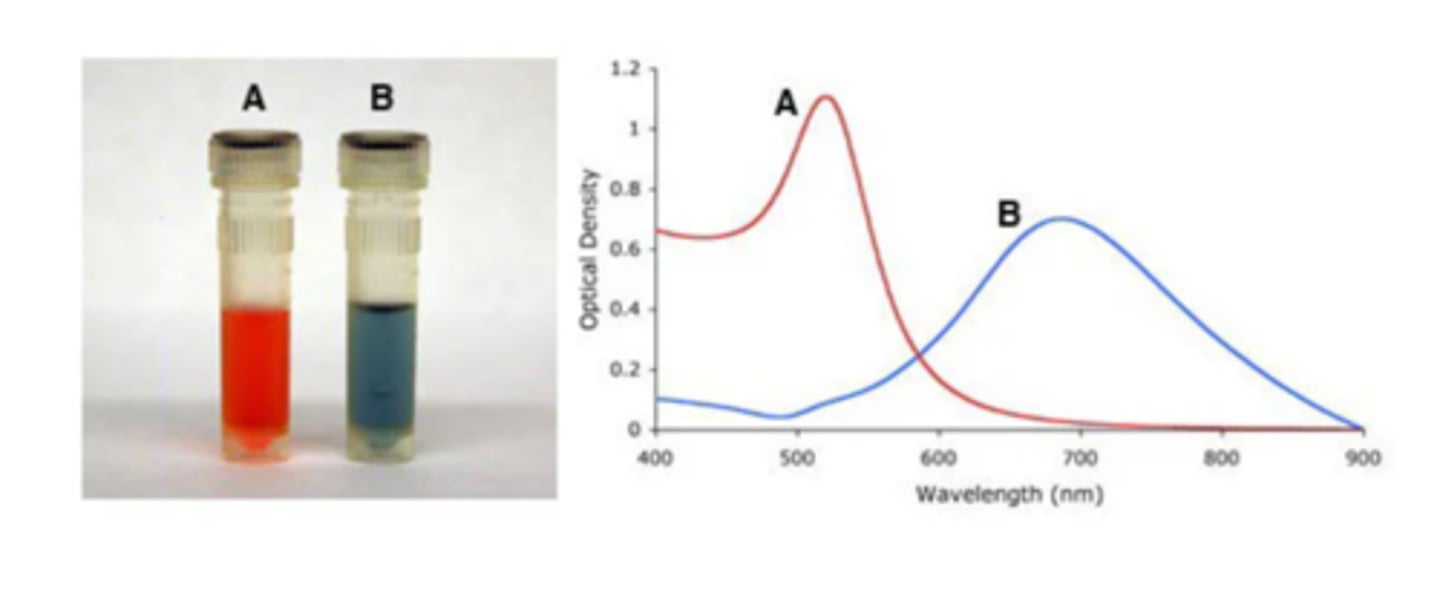
Explain the aggregation state of nanoparticles and surface plasmon band (2)
- surface plasmon band red shifts upon aggregation of nanoparticles due to interparticle coupling (shifts towards higher wavelength as particle size gets bigger)
- for 14 nm Au nanoparticles, the dispersion colour experiences red-to-blue transition during aggregation, which is widely used in Au nanoparticle-based colorimetric biosensors
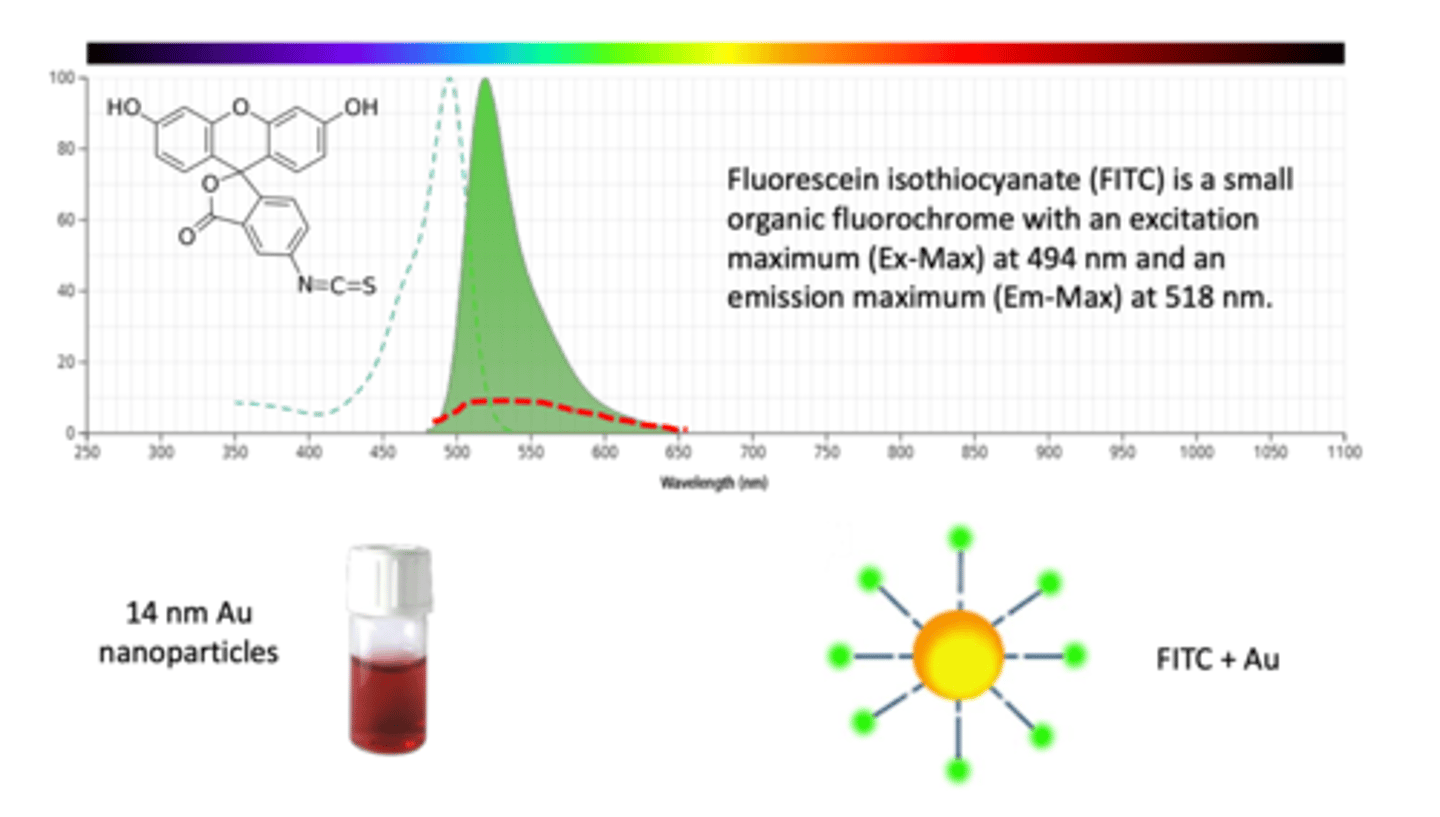
How do gold nanoparticles act as a quencher? (3)
- fluorescein isothiocyanate (FITC) is a small organic fluorochrome with an excitation maximum (Ex-Max) at 494 nm and an emission maximum (Em-Max) at 518 nm
- FITC is a fluorescent dye blue in colour
- when a gold nanoparticle is put together with FITC, they form a molecule and QUENCHES the colour of FITC (NO LONGER BRIGHT BLUE)
- beneficial when used to study biological interactions
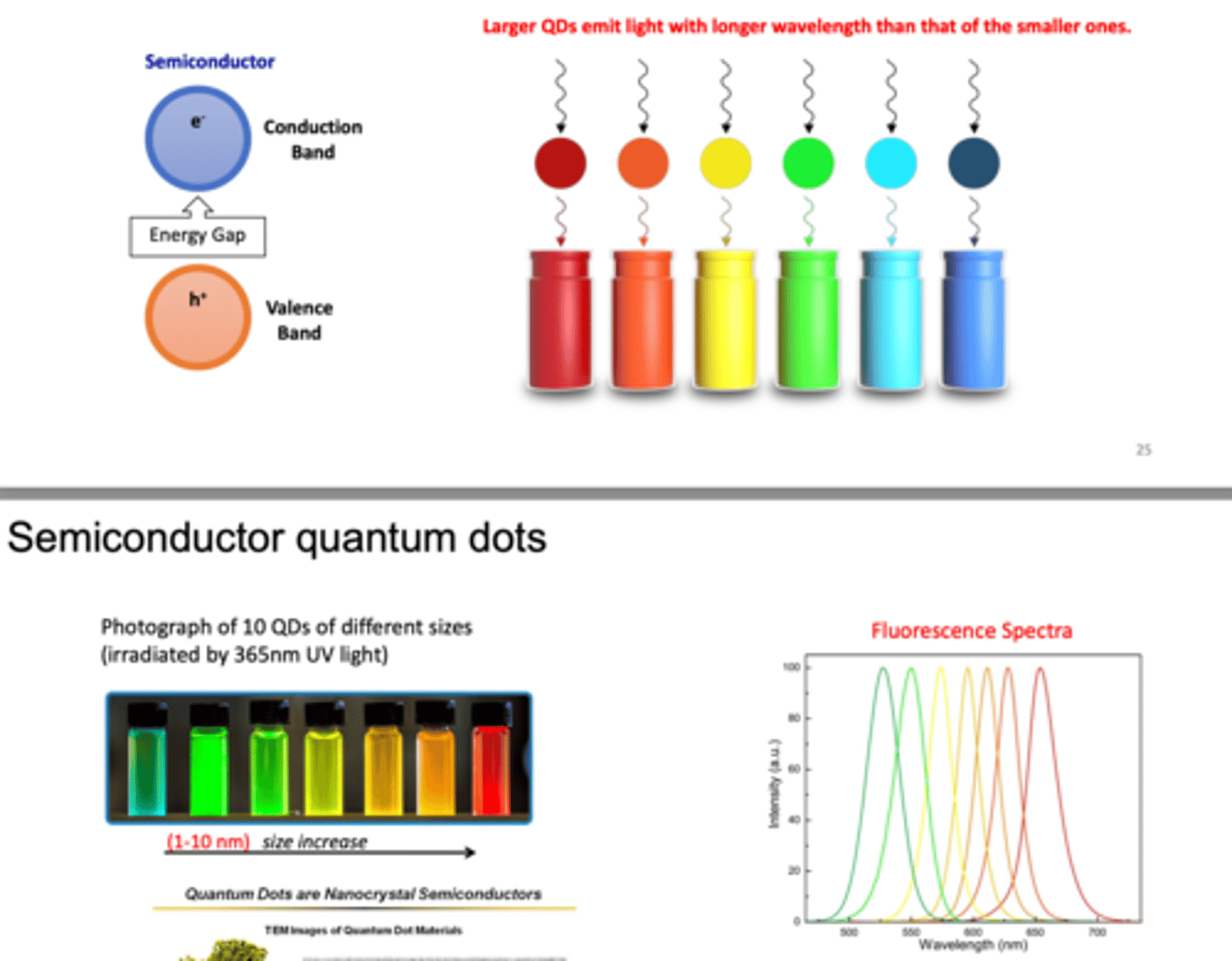
What are quantum dots? (3)
- nanocrystal made from semi-conducting materials
- the dots show quantum effects because they are so little
- this means that electrons inside the dot are trapped and can only occupy defined levels --> give off a certain colour
What is the applications of quantum dots in cell culture? (3)
1. when quantum dots are put into a cell's culture, due to its nanoscale, the cells will eat the QDs.
2. when a UV light (not visible to us) is shone into the culture, the QD will receive the rays and generate a bright colour --> cells can be VISUALISED EASILY
3. in quantum dots, we focus on light emission, not absorption as UV light absorbed by QDs is not visible light
How do semiconductor quantum dots work? (3)
- quantum dots with larger diameters emit light with longer wavelength (more red)
- semiconductors have the fully occupied valance band and unoccupied conduction band
--> with the small energy gap in between two bands, it takes a certain amount of energy to excite the electrons from the valence to conduction band
--> when electron jumps from valence to conduction band, they release different photons (different colours)
- photo below shows that we only need to buy a different QD size to have a different colour
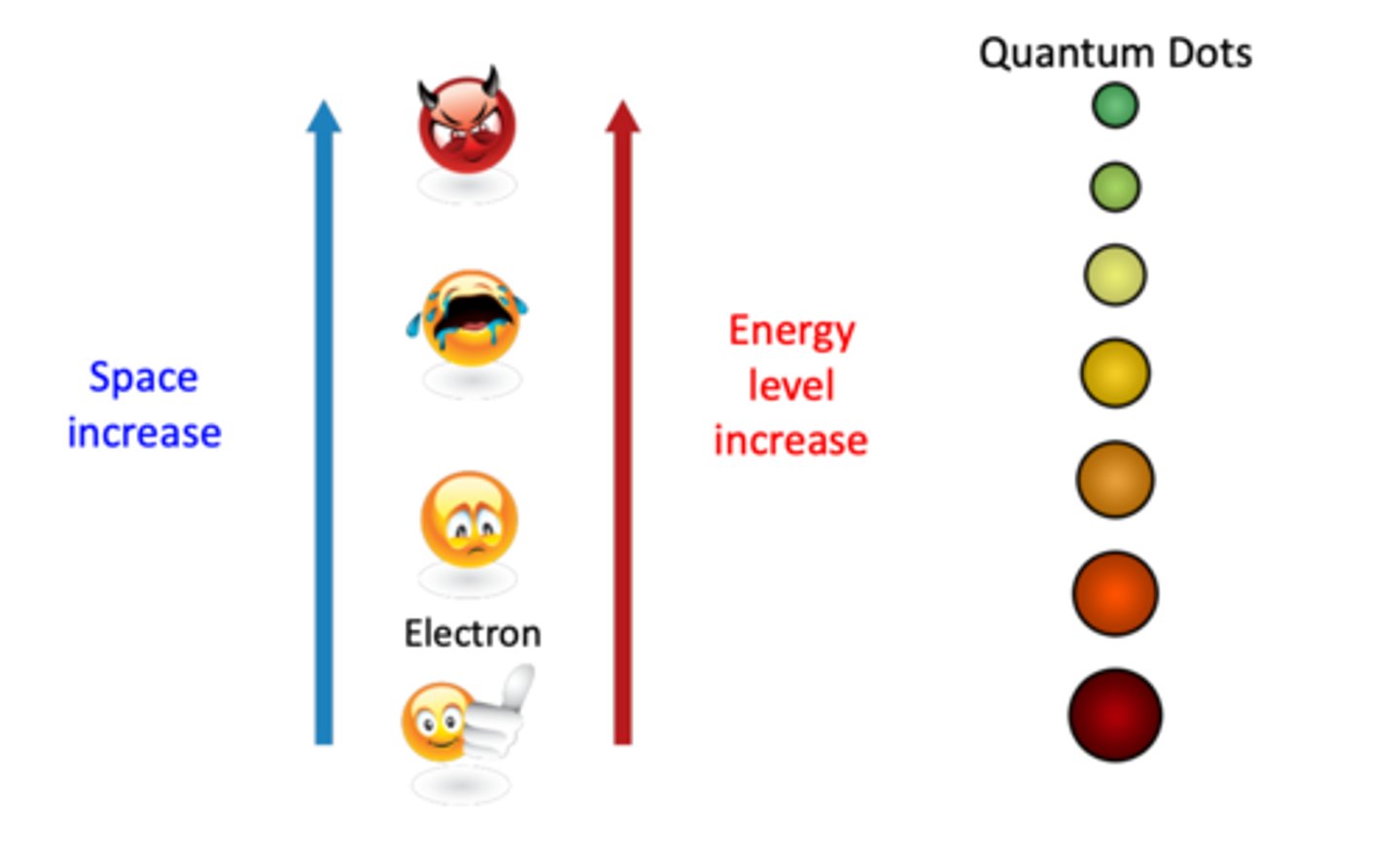
Define the quantum size effect (3)
- the band gap of QDs is size-dependent and decreases with increasing sizes
- smaller band gap corresponds to longer wavelength
- when size of the QD is smaller than the exciton bohr radius (electron & hole), energy band gap of the QD exhibits strong quantum size confinement and becomes size dependent
What happens when UV light is shone on electrons trapped inside quantum dots? (4)
- when electrons jump from a low energy state to a higher energy state, they will leave a hole in the valence band
- however, higher energy electron is not stable --> electron will jump back to the small hole where it originates from or to another slightly different energy state
--> when doing so, they give off light, this is the reason why we have colour spin
- light hat can excite smaller QDs also can excite larger QDs
- QD emission colour evolves from blue, green to red when its size increases
Example from diagram when electrons jump from energy states and emit photons
When blue light is given to QD, they become excited and jump energy --> then it becomes unstable to emit green photons to return to the valence band
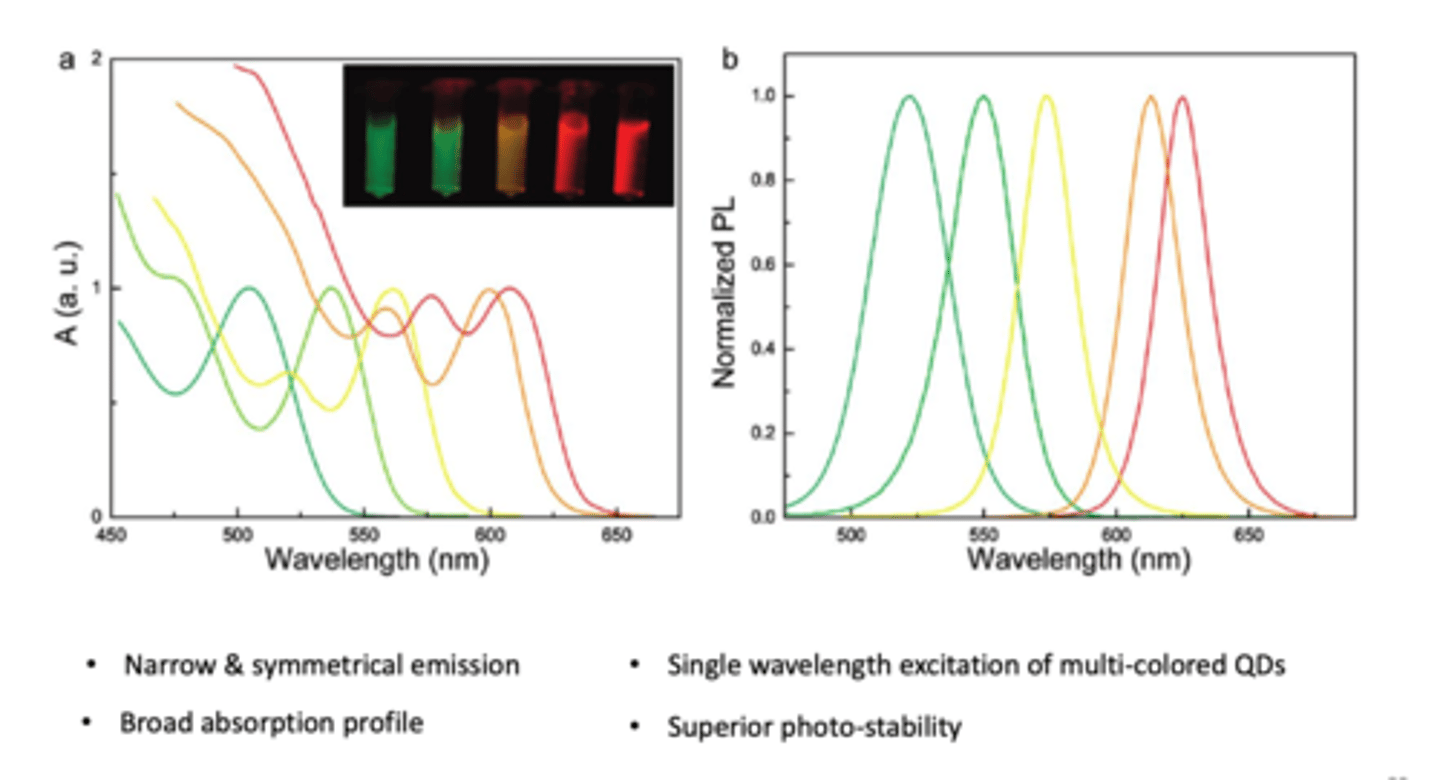
Optical properties of QDs (absorption and emission spectra) (4)
- QD gives narrow and symmetrical EMISSION
- BROAD ABSORPTION profile
- single wavelength excitation of multi-coloured QDs
- superior photo-stability
Why do we use UV light for QDs and why do we only use emission wavelength graphs? (2)
1. we use UV light for QDs because it is not visible, no colour and has a lot of energy
2. as a result, we can only see emission wavelengths --> visible and clear results, no interferences
What is a liposome (1965)?
liposomes are concentric bi-layered vesicles in which an aqueous volume is entirely enclosed by a membranous lipid bilayer mainly composed of natural or synthetic phospholipids
What is the purpose of liposome nanoparticles?
to target specific tissue e.g. cancer cells and to preserve drugs for delivery
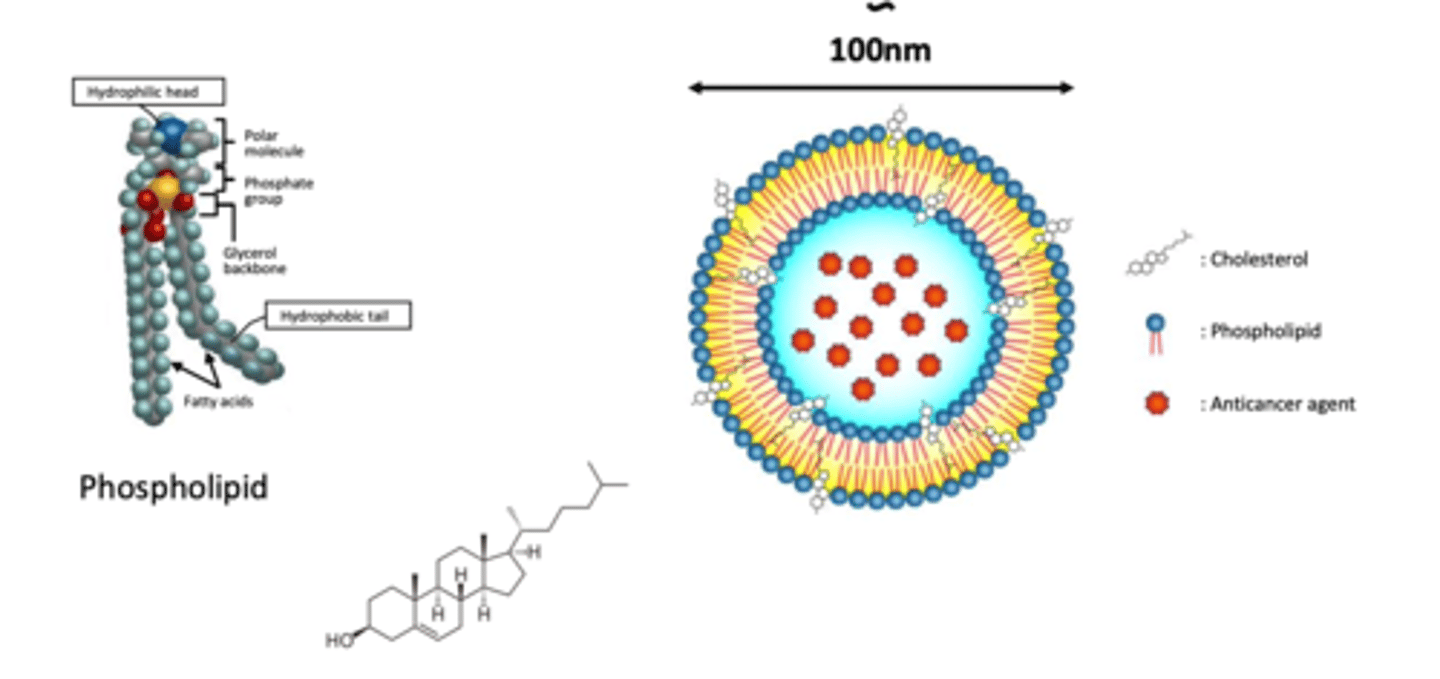
What is the role of cholesterol in structure of liposomes? (4)
- cholesterol is a molecule used to STABILISE structure of liposomes
- this is because the bilayer is not stable and can be destroyed in human's circulation and in high salt concentration environment
- exist in high concentration of up to 2:1 molar ratios of cholesterol to PC (phospholipid chain)
- hydroxyl group of cholesterol oriented towards the aqueous surface and aliphatic chain aligned parallel to the acyl chains in the centre of the bilayer
How are liposomes formed? (2)
- liposomes are formed when the thin phospholipid films are HYDRATED
- phospholipid bilayers spontaneously close in on themselves to form sealed compartments, which are called liposomes
Method of preparation of liposomes (2)
1. To make dry lipid film: lipid is dissolved in ethanol solution, solution is poured into Petri dish and ETHANOL dried off --> what's left = DRY LIPID BILAYER
2. When they are washed with an aqueous solution, they interact with WATER due to their hydrophobic properties and FORM LIPOSOMES
What is a critical step of liposome preparation? (2)
NOTE THIS IS HOW DOXORUBICIN IS MADE (bottoms-up synthesis method)
- Hydration agitation
- sonication
What is hydration agitation?
high speed spinning tool used to spin phospholipid film to spin it to disrupt membranes into particles
What is sonication?
disruption of cells by sound waves --> allows homogenisation into nanoparticles
Give an example of a liposome nanoparticle drug delivery
EXAMPLE: Doxil (Doxorubicin hydrochloride encapsulated in Stealth liposomes)
- 100 nm of carbonyl-methoxypolyethylene glycol (MPEG) 2000-1,2 distearoyl-sn-glycero...., fully hydrogenated soy phosphatidylcholine and CHOLESTEROL
- (from photo) doxorubicin is in the core of the liposome (hydrophilic)
- MPEG surface allows for surface modification so that the drug remains in circulation for longer

Current liposomal drug preparations
see table
*currently in clinical trials or approved for clinical use

What are the aims of the cancer vaccination concept? (2)
1. to identify the genetic material that is specific to the cancer cells --> if we know that protein specifically --> then we can make mRNA specific to it and inject into human's body to create antigens for the specific type of cancer
2. when the cancer emerges from within, the body is prepared to eradicate the cancer cell
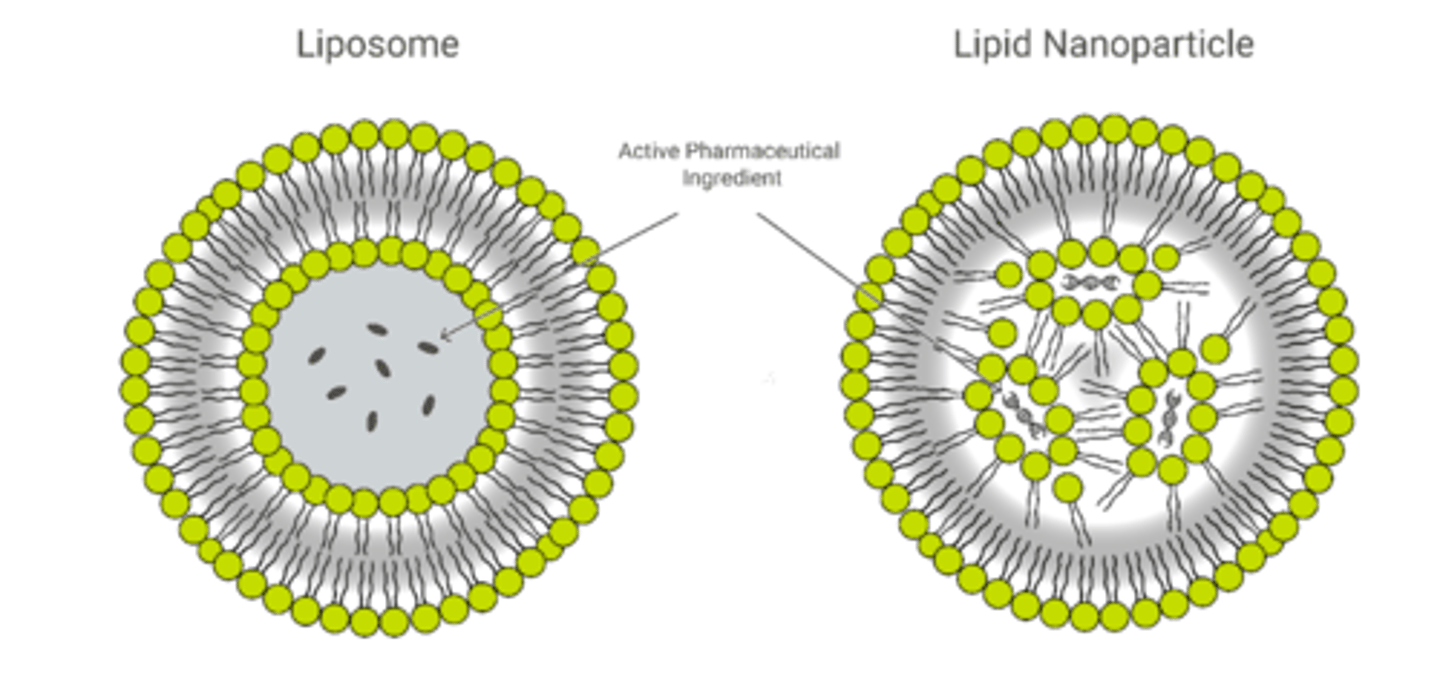
Features of liposomes (3)
1, spherical, symmetrical
2. formed mainly by phospholipids and other physiologic lipids
3. traditional liposomes include one or more rings of lipid bilayer surrounding an aqueous pocket
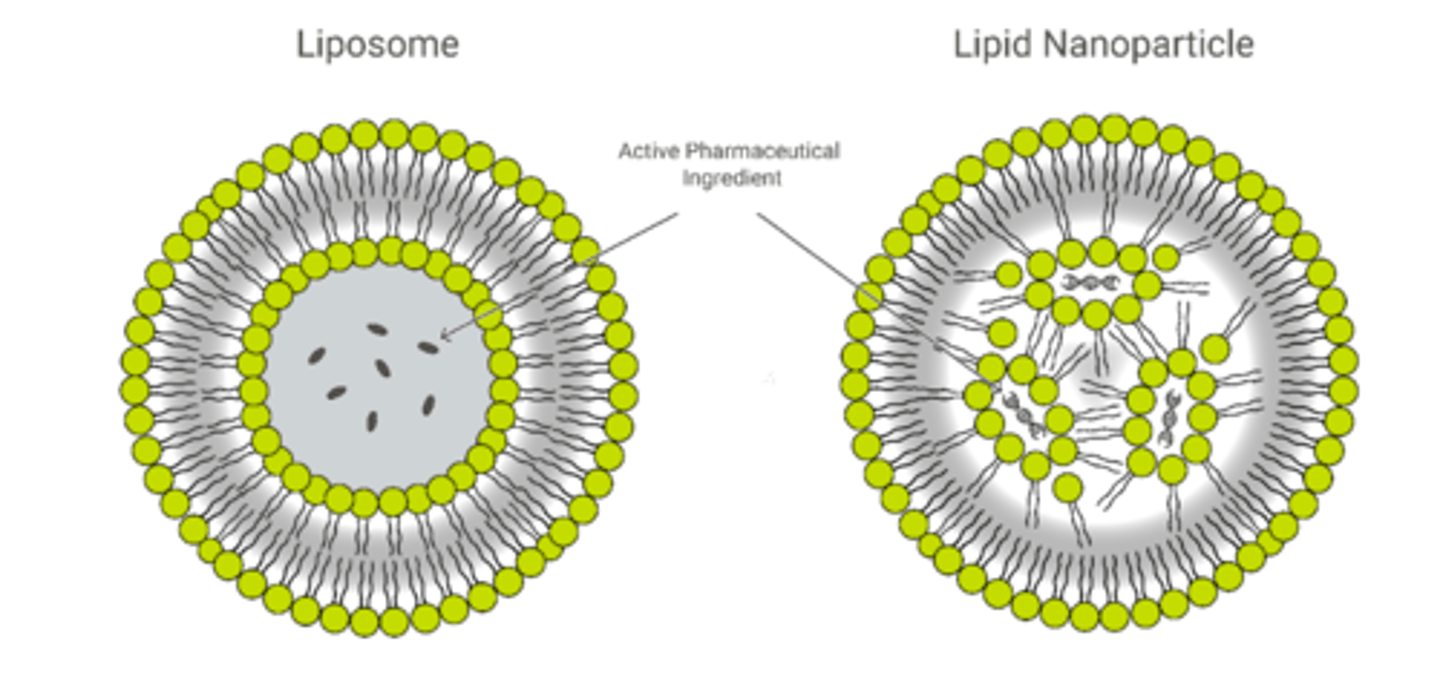
Features of lipid nanoparticles (5)
1. circular but does not form secondary circle
2. are solid particles at room and body temperature, consisting of solid lipids (SLN) or a mixture of a solid lipid and a liquid lipid (NLC)
3. formed due to interaction with active pharmaceutical ingredient (nucleic acids)
4. purpose is to encapsulate nucleic acids
5. not all LNPs have a contiguous bilayer that would qualify them as lipid vesicles or liposomes
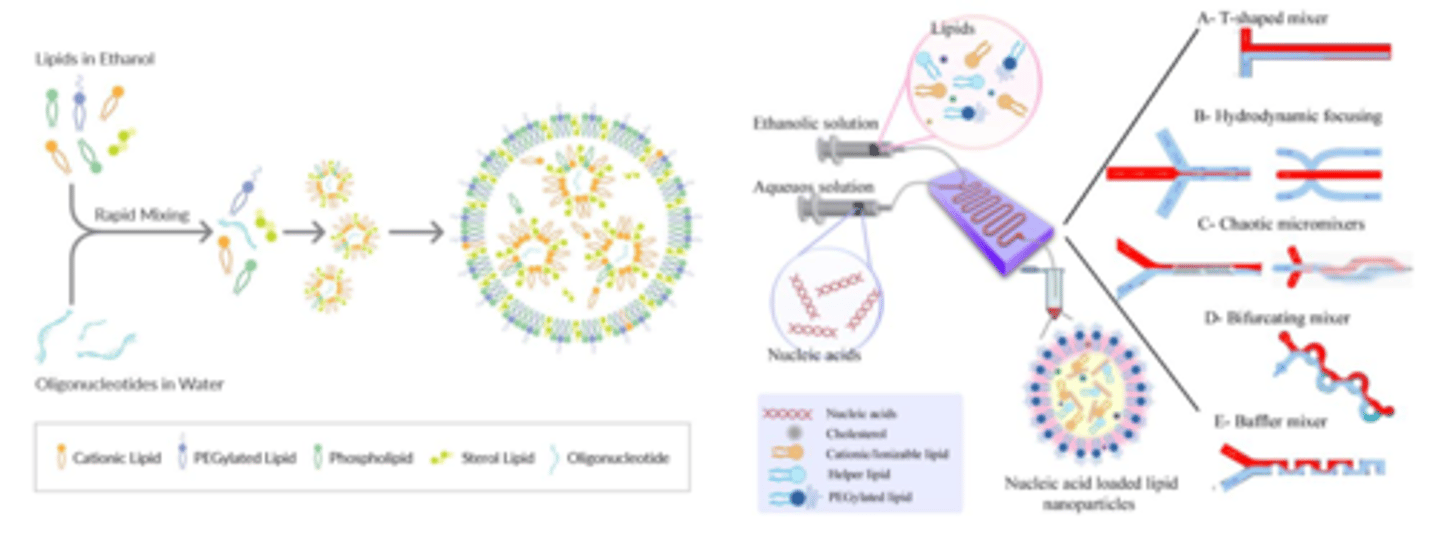
development of lipid nanoparticles using microfluidics (2)
1. ethanol solution containing ethanol and aqueous solution containing nucleic acids is put into microfluidic device and mixed together
2. forms nucleic acid loaded lipid nanoparticles
Example of lipid nanoparticle (encapsulate nucleic acids) in clinical use COVID-19 (2)
- Covid-19 vaccine is a nucleic acid (mRNA). However, if mRNA is injected directly into the human body, it will be eaten up by the immune system --> damaged
- aim of the vaccine is to tell the body to target the spike protein that is present in SARS-CoV-2
Explain features of nano-delivery system of mRNA Covid-19 vaccine (4)
- a fragment of spike gene nucleic acid is generated using biotechnology
- from photo: blue rods called spike mRNA is delivered into the cells using lipid nanoparticles
- once inside the cells, the mRNA will make spike protein --> triggers the body to recognise spike protein as an antigen --> train T-cells to recognise spike protein antigen
- this process takes LESS TIME because the protein antigen is made after injection into body's cells
Explain nano-delivery protein-based system Hep B (3)
- protein particles is prepared using microbe organisms
- genetic material (plasmid containing gene for hep B surface antigen) is inserted into yeast cells using electroporation
--> yeast is grown via fermentation and reproduce more surface protein
--> large quantity of protein particles is harvested, purified and used as vaccines
LENGTHY PROCESS COMPARED TO JUST MAKING mRNA
What are the 2 steps of nanoparticle fabrication?
- synthesis
- surface engineering
Nanotechnology is often represented by 2 fundamentally different approaches?
1. top-down
2. bottom-up
Explain the 'top-down' approach of nanoparticle fabrication
making nanoscale structures by machining, templating and using lithographic techniques (by extruding and sonicating)
Explain the 'bottom-up' approach or 'molecular nanotechnology' of nanoparticle fabrication
building organic and inorganic materials into defined structures, atom-by-atom, or molecule-by-molecule, often by self-assembly or self-organisation
List 3 methods of 'top-down' synthesis
1. nanoprecipitation
2. spray-drying
3. PRINT (Particle Replication in Non-wetting Templates)
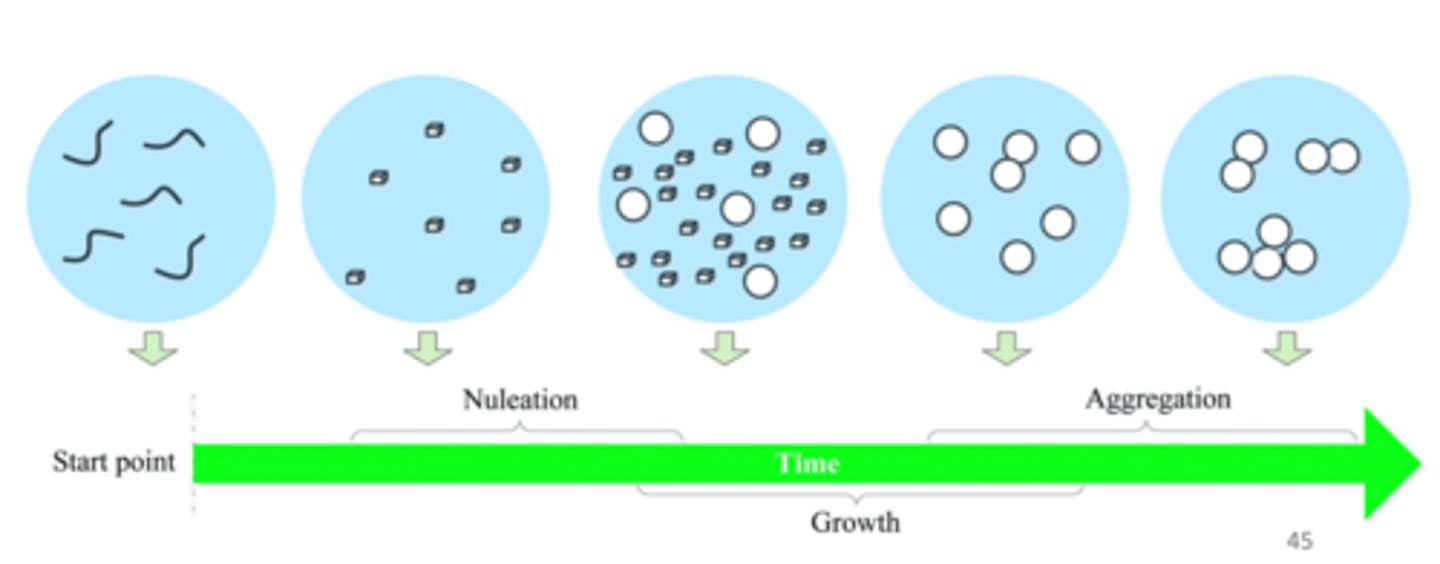
Describe features of nanoprecipitation BOTTOM-UP
starting point have lots of molecules --> forms into bigger molecules via nucleation --> continues to grow and aggregate to form larger particles
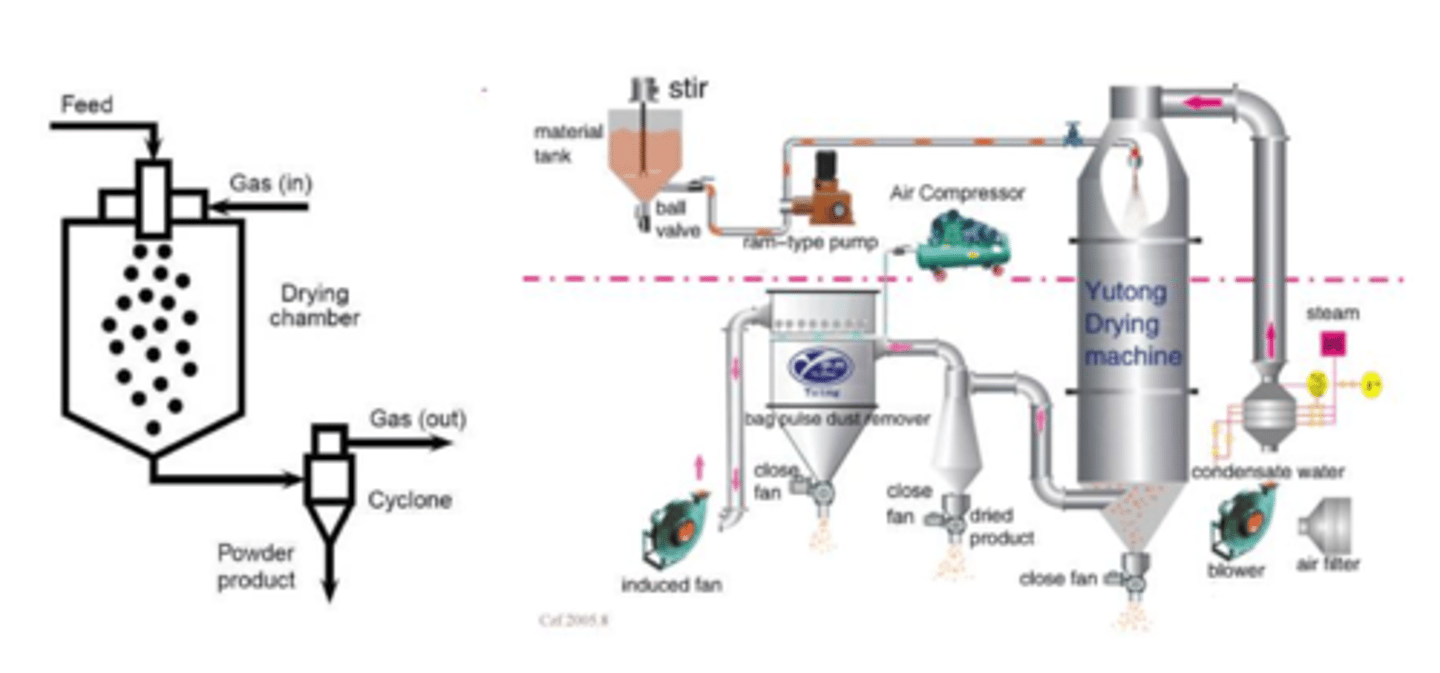
Describe features of spray-drying TOP-DOWN (3)
- important to make inhalations
- solution that contains material is delivered into a mixing cylinder where it is pumped with air and dried --> formed particle is collected at the end of the system
Why don't we make nanoparticle inhalants? (3)
- large particles too big and makes the particle stuck in the throat --> irritating
- however nanoparticles are not stable --> they aggregate and try to form bigger particle, also not easy to make
- if hypothetically we can make a stable nanoparticle inhalant then it would be very good
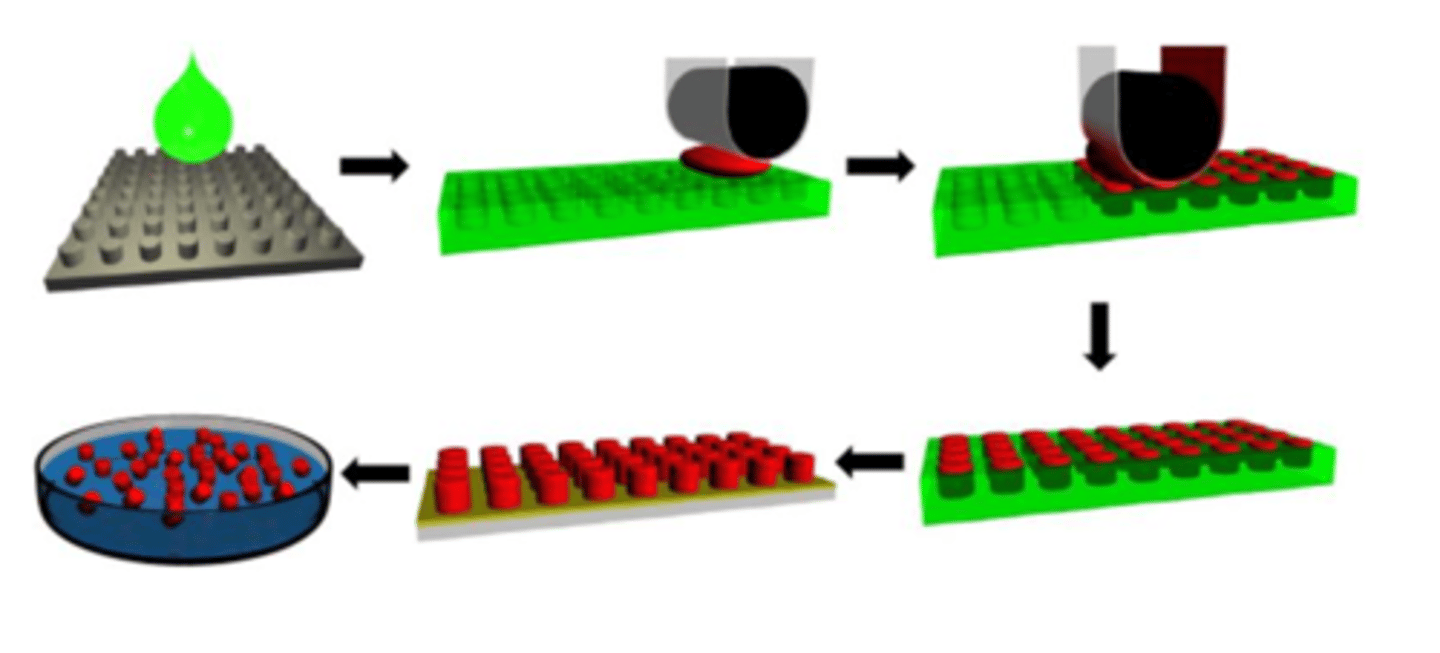
Describe the features of PRINT (Particle Replication in Non-wetting Templates) (5)
1. a drop of green liquid material is dropped onto a non-wetting template (grey), which serves as a mould
2. the green liquid will then form micro-wells --> forming a micro-mould
3. red colour is the particle you want to make, when it is spread onto the green template using a roller, they form micro-particles onto the moulds
4. the green colour mould is then dissolved using organic solvent to collect red particles
5. *NICELY CONTROLLED PROCESS: produce uniformed particles due to them being made in templates
Describe a method of 'BOTTOM-UP' synthesis SELF-ASSEMBLY
SELF-ASSEMBLING DELIVERY SYSTEMS
- the next advance was to construct materials that would self-assemble with drugs to create controlled drug delivery vehicles
- self-assembly is the typical approach using a molecule that has a hydrophilic head and hydrophobic tail to form a shell
- starts with smaller molecules nucleation that forms aggregation and oriented attachments --> snowflake formation due to electronic interaction between molecules (orient a certain way)
- self-assembly: hydrophilic stays together, hydrophobic stays together
*LIPOSOMES*
Describe the process of ruby red colloidal gold (3)
1. citrate ions is added into gold nanoparticles to form colloids when put under heat
2. to test whether or not colloidal gold nanoparticle has been formed, we utilise the Tyndall effect
3. when a laser beam is shone through the solution, if the solutions show the light path of the beam --> that means gold nanoparticles has been formed (left)
*compared red with solution on the right, there is no light beam seen in the solution --> no gold colloid particle

How is graphene involved in both the bottom-up method and top-down method? (2)
1. bottom-up method: there are carbon molecules to start with --> precipitated to form graphene sheets
2. top-down method: start with graphite and removes graphite layer by layer using scorch tape
why do we use graphene in nanoparticle systems? (2)
- to make fullerene and nanotube for drug delivery and application
- however, graphene can be toxic, compromise function and lead to cell death --> balance between efficacy vs toxicity
What are the applications of surface particle modification? (5)
- water-solubility
- colloidal stability
- surface-functionality
- nanoparticle-cell interaction
- bio-distribution and pharmacokinetics
Stabilising mechanism for nanoparticles in aqueous medium (4)
- hydrophilic thiol coating
- silica coating
- amphiphilic polymer coating
- PEG coating
Explain the role of PEG coating in surface modification of nanoparticles (3)
- polyethylene glycol (PEG) can be a very good disintegrant
--> very hydrophilic
- low molecular weight (MW) PEG is in liquid and high MW PEG is solid
- when injected into human body, PEG increases circulation time being very water soluble and evade attack from human cells due to its non-familiar structure (man-made molecule)
Explain the way PEGylation reduces non-specific binding in nanoparticle delivery (3)
- PEGylation: the process by which poly(ethylene glycol) chains are attached to carriers
- the ability of PEG to reduce non-specific protein binding is believed to result from the preferred (polar) gauche conformation of PEG in water, which offers two hydrogen acceptors in ideal distance for hydrogen bonding with water
- this conformation leads to extensive hydration in aqueous environments which, along with good conformational flexibility and high chain mobility, causes a steric exclusive effect that prevents the adsorption of proteins
Why are charge-stabilised nanoparticles desired? (5)
- particles in a solution that go through sonication and extrusion will have friction, which will result in charge (negative or positive)
- aim is to have charged particles
- neutral particles are not good as they form aggregates
- repulsion of surface charges on nanoparticle surface prevents the nanoparticles to aggregate
- increase of salt concentration leads to thinner electrical double layer and thus reduced electrostatic repulsion (poorer colloidal stability)
How does polymer coating increase stability of particles? (5)
POLYMER LIGANDS: steric stabilisation (briefly went through)
- polymer coating will increase stability of particle --> can't be pushed easily by other particles
- as the distance of separation between the particles decreases in the coagulation step --> the polymer coatings begin to overlap, which is entropically unfavourable, and thus push the nanoparticles away from each other
- ultimately, it is the crowding of the polymer chains within this overlap volume that produces the steric stabilising effect
- polymer ligands should have good solubility in aqueous medium
- higher graft density of polymer ligands leads to better stabilisation (stronger steric stabilising effect)
What is the challenge posed by protein corona on nanoparticles? NON-SPECIFIC BINDING (2)
- nanoparticles when exposed to biological fluid, become coated with proteins and biomolecules to form a 'protein corona' due to nanoparticles being charged
- non-specific binding forms a monolayer on nanoparticle surfaces and has micromolar affinity, and this non-specific binding can be detrimental to the built-in molecular recognition in the nanoparticles --> this is NOT GOOD because WE WANT IT TO REMAIN SMALL
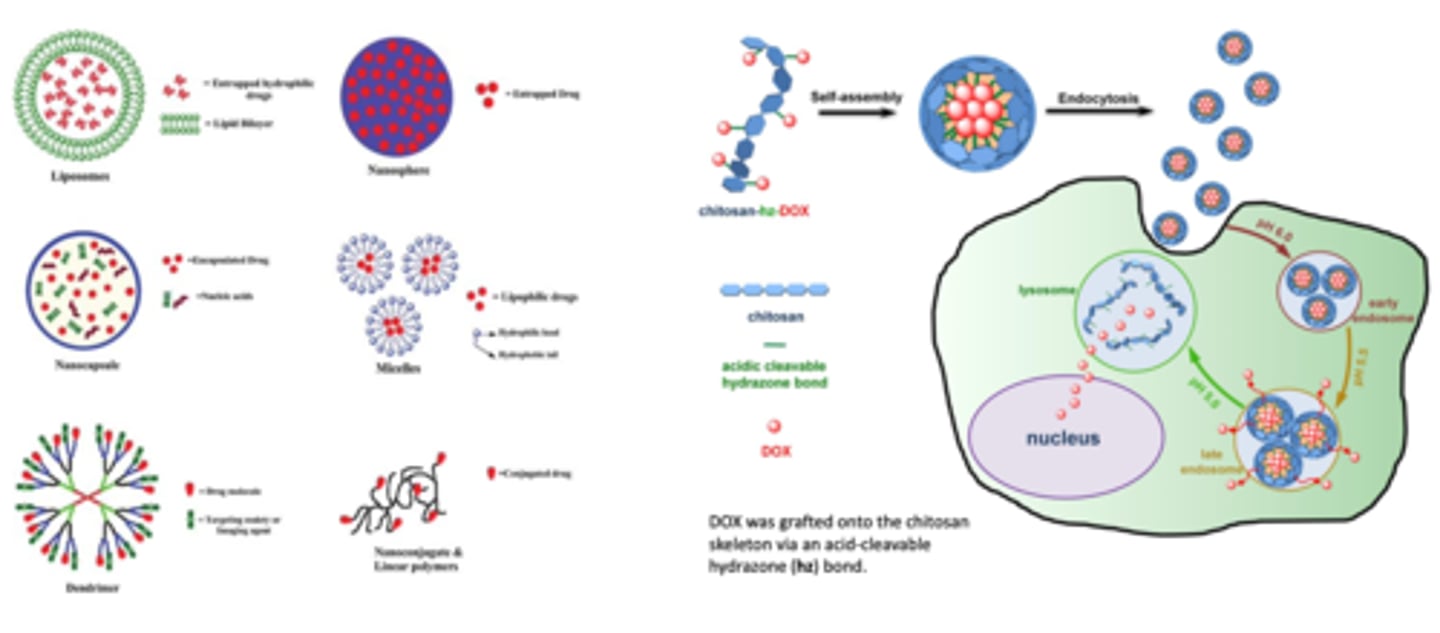
How does doxorubicin get released in the body? NANOMEDICINE DRUG DELIVERY (3)
1. Doxorubicin chemically CONJUGATES to the CHITOSAN molecule --> self assembly into liposome
2. nanoparticle is then injected into the bloodstream and absorbed via ENDOCYTOSIS
3. ph change causes drug release
Give an example of how nanocarriers play a role in cancer therapy OSTEOSARCOMA
- peptide VATANST (STP) can specifically bind with VIMENTIN --> highly expressed on the osteosarcoma cell surface
- nanoparticle is core-shell structured to protect the loaded DOX during blood flow
- DISULFIDE BONDS within the nanoparticles are SENSITIVE to the osteosarcoma microenvironment *microenvironment has HIGH GLUTATHIONE (GSH) levels*
- under the ENHANCED PERMEABILITY and retention and ACTIVE TUMOUR TARGETING EFFECTS --> the STP-decorated DOX-loaded NPs accumulated in tumour tissues
- high GSH levels can RUPTURE DISULFIDE BONDS, resulting in the CONTROLLED RELEASE of DOX --> causing necrosis of tumour cells
*nanoparticles could increase the tumour inhibition efficiency against osteosarcoma and reduce the side effects of DOX to major organs*
Differences between ADME (3)
ABSORPTION: small molecules are absorbed through passive diffusion whereas nanoparticles are absorbed through endocytosis
DISTRIBUTION: distribution is everywhere for normal particles BUT for nanoparticles --> you can make them specific to target tissue (tumour)
METABOLISM: slower release for nanoparticles --> controlled release. ALSO --> nanoparticles have LESS toxicity because they are absorbed in SPECIFIC TISSUE --> LESS SYSTEMIC EFFECT
How does the size of nanoparticles affect biodistribution? ENHANCED TARGETING (3)
- particles <10nm are 400x more likely to cross the intestinal wall than 1-micron particles
- particles between 1- and 10-micron deposit in the deep lung via impaction while other escape during breath or deposit in the mouth
- particles below 500nm can escape the filtering of the liver and kidney for several cycles
How can nanoparticles affect cellular interaction?
nanoparticles are highly charged with high surface to volume ratio --> this can affect CELLULAR INTERACTION
What is the particle size range of nanoparticles?
10 nm to 200 nm
List the advantages of nanoparticles for drug delivery (5)
- improved bioavailability by enhancing aqueous solubility
- suitable for intravenous delivery
- increase circulation time in the body
- extravasate through the endothelium in inflammatory sites, epithelium (e.g. intestinal tract and liver), tumours or penetrate microcapillaries
- allows for efficient uptake by a variety of cell types and selective drug accumulation at target sites
Naked drugs vs formulated drugs with nanoparticles (3)
- formulated drugs can cross the cell membrane easier
- formulated drugs have better targeting/specificity to tissue and cells
- formulated drugs have better drug protection and decreased toxicity
List the sequential biological barriers to nanoparticles (6)
- mononuclear phagocyte system
- nonspecific distribution
- haemorrheological limitations
- intratumoral pressure
- cell membrane/internalisatoin endosomal escape
- mulidrug resistance
Explain the mononuclear phagocyte system NANOPARTICLE BIOLOGICAL BARRIER
macrophages absorb nanoparticles --> can't reach target
Explain the non-specific distribution NANOPARTICLE BIOLOGICAL BARRIER
some non-specific molecules could end up in the liver --> we don't want this
Explain the haemorrheological limitations NANOPARTICLE BIOLOGICAL BARRIER
circulating in the system but not making contact with epithelial cells
Explain intratumoral pressure NANOPARTICLE BIOLOGICAL BARRIER
- tumour tissue has high pressure --> difficult for nanoparticle to penetrate tissue
Explain how multi-drug resistance can occur in nanoparticles NANOPARTICLE BIOLOGICAL BARRIER (2)
- efflux system pushes drug particles out
- tumour cells are similar to bacteria
Explain how nanoparticle design can overcome delivery barriers
1. Different nanoparticle sizes can have different retention in different organs:
- < 5nm = deposit in the kidneys
- >150 nm deposit in the liver/lungs/spleen
2. Different nanoparticle shapes
- circular shape has higher concentration in the lungs/liver/spleen
3. Different charged particles
- positively charged particles have highest concentration in the liver

What is the principle of PLGA PRINT particles? (2)
- make different nanoparticles
- once shape and size is created --> load nanoparticle into a SPECIFIC tissue
Describe the fluorescence phenomenon
if we introduce a gene into a cell --> it can fluoresce and help us visualise microbial cells
Give an example of gene therapy CYSTIC FIBROSIS
- normal CFTR channel = moves chloride ions to the outside of the cell
- mutant CFTR channel = does not move chloride ions, causing sticky mucus to build up on the outside of the cell
TREATMENT: deliver cDNA od Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) to epithelial tissue of respiratory system
What are the 2 types of gene delivery vectors? (2)
- viral
- non-viral
List the VIRAL gene delivery vectors (4)
DNA is inserted into a virus which is then sent to a target cell:
- retrovirus
- adenovirus
- adeno-associated virus
- herpes simplex virus
List the NON-VIRAL gene delivery vectors (4)
DNA in a circular plasmid is sent into a target cell:
- liposome
- polymers
- naked DNA --> insert genes of interest into plasmid and transfer into cells
- physical methods
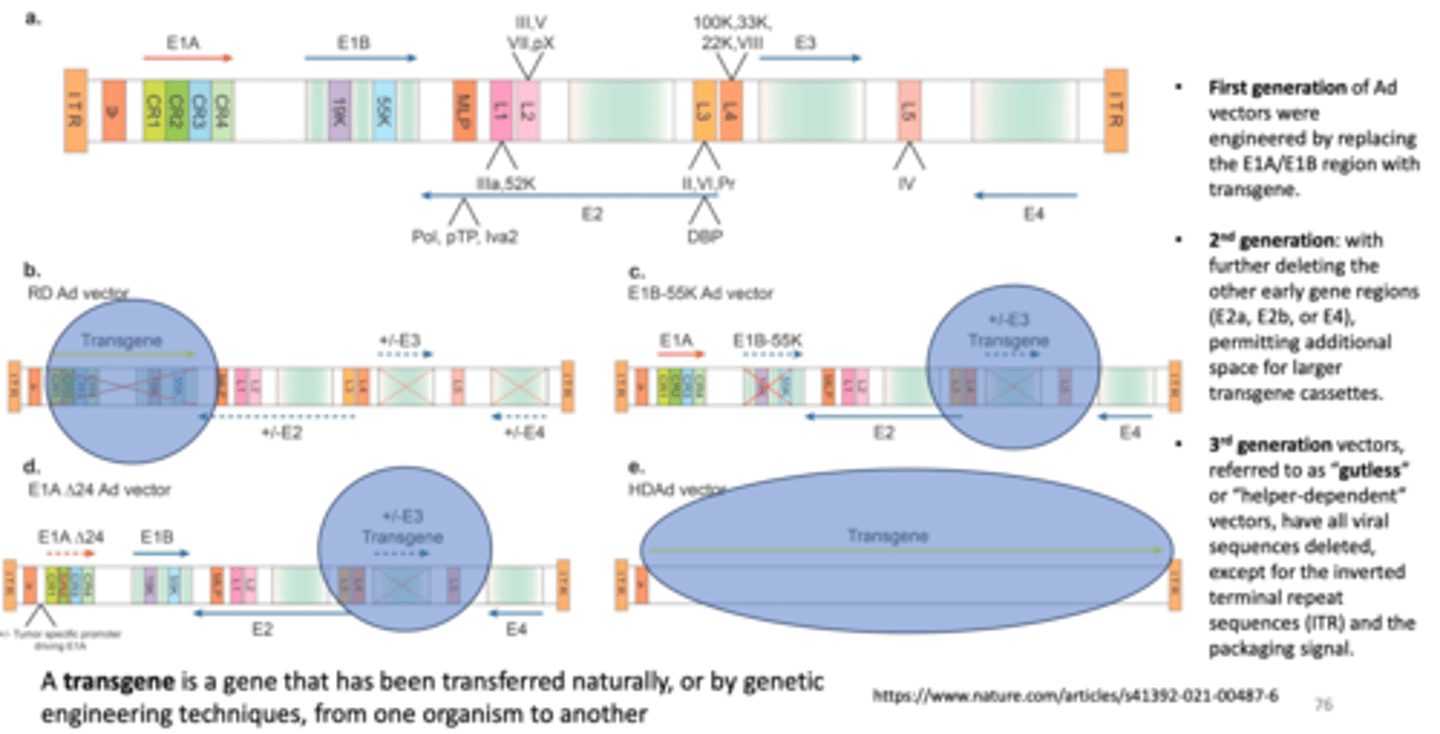
Define what a transgene is
a gene that has been transferred naturally, or by genetic engineering techniques, from one organism to another
Describe the process of inserting transgenes into adenovirus in FIRST GENERATION ADENOVIRUS VECTORS
Ad vectors were engineered by replacing the E1A/E1B region with transgene
Describe the process of inserting transgenes into adenovirus in SECOND GENERATION ADENOVIRUS VECTORS
with further deleting the other early gene regions (E2a, E2b, or E4), permitting additional space for LARGER transgene casettes
Describe the process of inserting 3rd generation vectors
- also referred to as "gutless" or "helper-dependent vectors, have all viral sequences deleted, except for the inverted terminal repeat sequences (ITR) and the packaging signal
Describe in vivo gene therapy
the direct administration of vector carrying a therapeutic transgene into the patient
Describe ex vivo gene therapy
involves the extraction of a patient's cells or from an allogenic source, genetic modification by a vector carrying a therapeutic transgene, selection and expansion in culture, and infusion to re-introduce the engineered cells back into the patient
What are the 4 gene therapy approaches? (4)
- gene replacement
- gene silencing
- gene addition
- gene editing
Define gene replacement therapy
the delivery of a functional gene to replace a non-working gene
Define gene silencing
inactivation of a mutated gene that has become toxic to cells
Defene gene addition
over-expression of a "foreign" or exogenous gene to impact cellular function
Define gene editing
a PERMANENT manipulation of a gene in a patient's genome
What are the 3 components of a viral vector?
1. the PROTEIN CAPSID and/or envelope that encapsulates the genetic payload, and defines the vector's tissue or cell tropism and antigen recognition
2. the TRANSGENE of interest, which when expressed in cells, serves to confer a desired effect; and
3. the "regulatory cassette," the combined enhancer/promoter/auxiliary elements that controls stable or transient somatic expression of the transgene as an EPISOME or as a CHROMOSOMAL INTEGRANT
Adeno-Associated Viral (AAV) vectors are usually what?
non-integrating
What are the features of non-integrating vectors? (3)
- DNA that is carried doesn't typically insert itself into the cell's genome
- So, if the vector is taken up by a cell that divides, the therapeutic gene won't be copied with each cell division and may be lost over time --> DILUTING THE TREATMENT EFFECT
- therefore, AAVs are commonly used to target NON-DIVIDING CELLS such as cells in the liver, nervous system, eyes and skeletal muscles
*AAVs can persist in patients for a prolonged period - possibly even a LIFETIME --> adenoviral vectors are like AAV vectors
What are the features of integrating vectors?
1. (examples) lentiviral and retroviral vectors can carry larger genetic packages of RNA which is converted intoDNA
2. during this process, the vectors INTEGRATE into the genome of the target cell, unlike AAVs and adenoviral vectors
3. the ability to integrate into the cell genome makes lentiviral and retroviral vectors are best suited for DIVIDING CELLS --> targets of EX VIVO TREATMENT approach
4. EX VIVO treatment from integrating vectors --> vectors are used to target T cells which are a type of immune cell and stem cells which are special cells that can develop into many cell types
Outlne the first FDA-approved gene therapy (4)
1. inside eye, the RPE65 gene provides instructions to make a protein for NORMAL VISION
2. people with RPE65 gene mutation cannot make the protein
3. Luxturna (voretigene neparvovec-rzyl) loads normal genes (a DNA strand) into a vector, an inactivated AAV2 virus
4. the vector, injected into the eye, delivers the normal RPE65 gene to eye to make the protein to restore eyesight
*GENE DOES NOT GET INCORPORATED INTO THE GENOME*
(non-integrating vector)
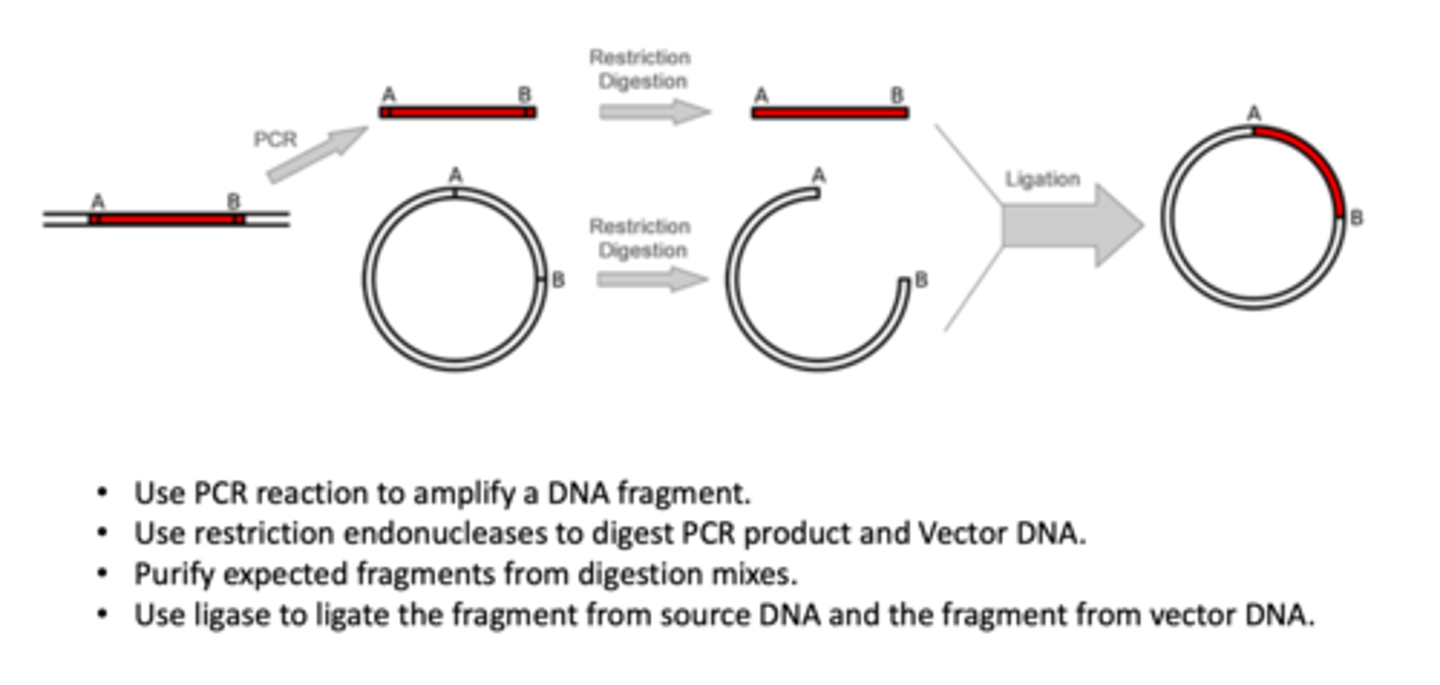
Describe the process of molecular cloning (4)
1. use PCR reaction to amplify a NDA fragment
2. use restriction endonucleases to digest PCR product and vector DNA
3. purify expected fragments from digestion mixes
4. use ligase to ligate the fragment from source DNA and the fragment from vector DNA.
*step 4 involves cutting circular plasmid and adding DNA fragment to circular plasmid
Examples of NON-VIRAL vectors PHYSICAL METHODS (4)
- gene gun
- electroporation
- magnetic field
- light-induced delivery
Explain the use of gene gun as a NON-VIRAL vector (3)
- shooting high velocity nanoparticles via mechanical force
- high velocity will allow for penetration into cell membrane
- cannot use needle syringe because it doesn't have a high enough velocity to reach the cell
used to make genetically modified organisms (GMOs)
Explain the NON-VIRAL gene delivery CHEMICAL METHODS (3)
1. negatively charged nucleic acid (DNA or RNA) is mixed with a cationic polymer to form polyplex particles which can enter target cells by endocytosis
2. polymer promotes the release of polynucleotide from the endosomes and the entrance of DNA to the nucleus while protecting the target nucleic acid from lysosomes degradation
3. pH change inside the cell allows for controlled-release of drug
Give an example of a cationic lipid-based delivery system (2)
1. Lipofectamine consists of a 3:1 mixture of DOSPA and DOPE
2. they complex with negatively charged nucleic acid molecules to allow them to overcome the electrostatic repulsion of the cell membrane