ISOMERS
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
Isomers
same molecular formula but different structure
Constitutional Isomers
different connectivity
skeletal
positional
functional
Skeletal Isomers
are isomers with similar formula but different arrangement of carbon atoms.
branching
Positional Isomers
are isomers with similar formula and functional group but at a different position.
locant
Functional Isomers
are isomers with similar formula but different functional group.
Stereoisomers
two or more molecules differing only at the spatial arrangement of their atoms
same connectivity
conformation
configuration
Conformation
Any spatial arrangement atoms may adopt and convert into thru rotation about individual single bonds
e.g. Sawhorse, Newman, and Ring conformations.
arrangements that result from the rotation around a single bond
Representation of Conformations
Sawhorse conformation
Newman Projection
Ring Conformation
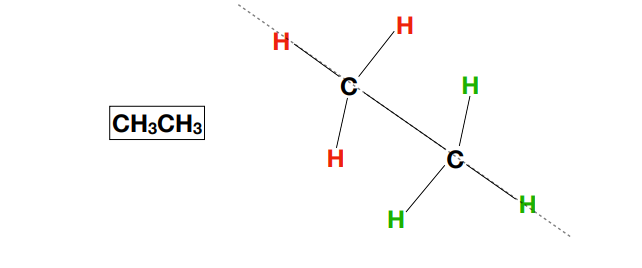
sawhorse conformation
newman projection
what can a molecule exhibit in case of steric effect?
molecules exhibit torsional strain
Torsional Strain
bending strain exhibit in the bond between the C’s at the point of rotation
Ring conformation
shows the arrangement of carbon molecules in a cyclic / ring form
Angle strain
results from the angular deviation of ring structures from the normal 109.5℃ angle of each carbon bond
results into higher energy and more reactivity of molecules with angle strain
cyclohexane
has the most stable ring conformation
does not produce an angle strain
boat or chair
Configuration
It is a fixed 3D relationship of atoms in a compound as a results of the bonds between them
e.g. Optical and Geometric configurations.
Optical Configuration
It is based on the surrounding bonds attached to a chiral carbon
has optical activity measured by polarimeter
chiral carbon
carbon with 4 different substituent
Enantiomers
two compounds with same number and kind of atoms and bonds but differ at the spatial arrangement of the atoms and are non-superimposable mirror image of each other.
Diastereomers .
are two or more compounds which have different configurations at two or more stereocenters and are not mirror images of each other
Geometric Configuration
two or more compounds which differs from each other in the arrangement of groups in reference to a double bond, ring, or other rigid structure.
TYPES OF GEOMETRIC CONFIGURATION
cis-trans isomers
E-Z isomers
cis-trans isomers
isomers with same connections but differ in the spatial orientation of the group of atoms
used for disubstituted alkenes to illustrate the double-bond geometry of the alkene
cis
- indicates that the groups are attached “on the same side”
trans
- indicates that the groups are attached “across” each other
E-Z system
used to illustrate double-bond geometry for alkenes which are trisubstituted or tetrasubstituted
It makes use of the Cahn-Ingold-Prelog rules to assign priorities to substituents to determine the geometry of the double-bond.
E
- “entgegen” meaning “opposite”
Z
- “zusammen” meaning “together”
Cahn-Ingold Prelog Rules
Take the carbons in the double bond separately, RANK BY PRIORITY (atomic number) the atom directly attached to each carbon
If there is no difference in priority in the first atom, continue with the 2nd, 3rd, 4th and so on, until difference is found.
List the groups attached to each atom bonded directly to the atom attached to the stereocenter.
The list follows the prioritization based on atomic number (highest tp lowest), then compare and find the difference
Multiple-bonded atoms are equivalent to the same number of single-bonded atoms
R and S system
used to illustrate specific configuration of substituents around a chiral center
follows the same rules applied to E-Z system.
R
“rectus” = to the right = clockwise
S
“sinister” = to the left = counter=clockwise