CH166
1/162
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
163 Terms
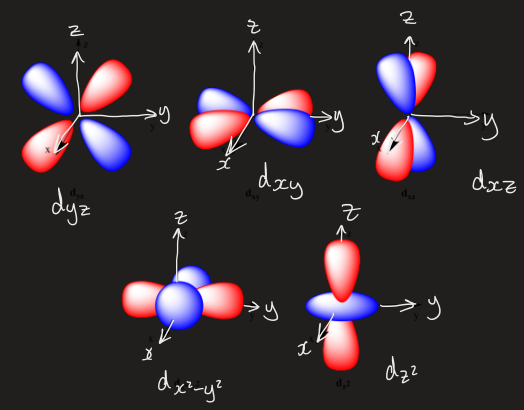
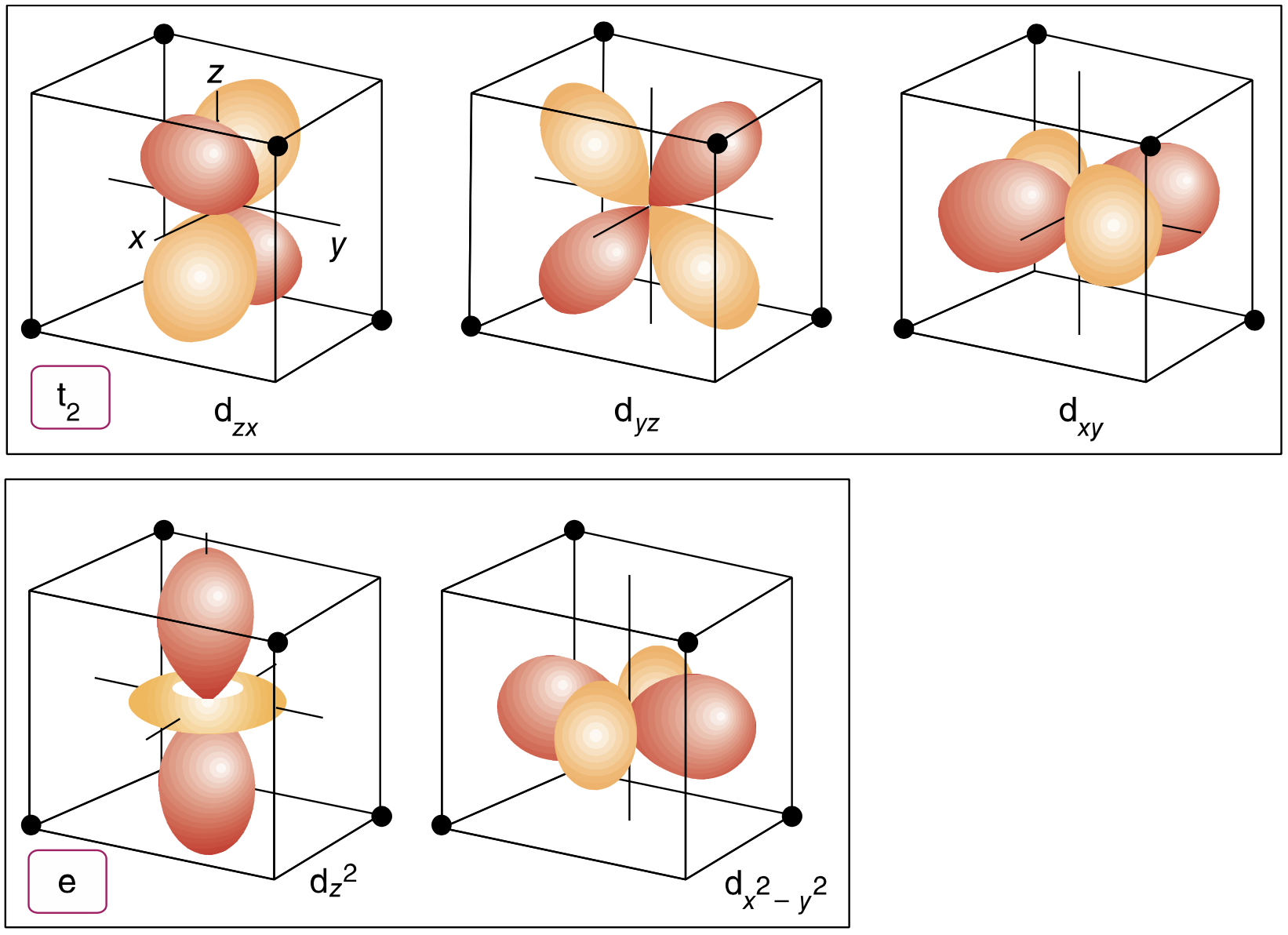
draw the d orbitals and label them
how do you know how many d electrons a transition metal has
number of d electrons = group number - charge
what are the general rules for crystal field theory
only considers electrostatics
ligands are like point charges
d orbitals split in response to the symmetry of the crystal field
draw the splitting pattern for octahedral symmetry
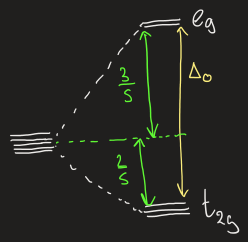
in octahedral symmetry which orbitals are eg vs t2g
eg:
dz2
dx2 - y2
t2g:
dxy
dxz
dyz
what is the equation for the crystal field splitting parameter
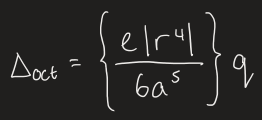
e = charge on electrons
q = charge on ligand
r = average d-orbital radius
a = metal to ligand distance
what are the factors affecting ∆oct
for the metal:
size of orbital
3d < 4d = 5d
increases with increasing O.S. of metal
bond length decreases with increasing metal O.S.
Mn2+ < Ni2+ < Co2+ < Fe2+ < V2+ < Fe2+ < Co3+ < Mo3+ < Rh3+ < Ru3+ < Pd4+ < Ir3+ < Pt4+
for the ligand
I- < Br- < SCN- < Cl- < ONO- < N3- < F- < OH- < C2O42- < O2- < H2O < NCS- < CH3CN < py:N < NH3 < en:N < bpn:N < phen:N < NO2- < PPh3 < CN- < CO
weak field ligands
I-, Br-, SCN-
prefer low spin
large ∆o
strong field ligands
CO, CN-
prefer high spin
small ∆o
what tends to high spin vs low spin
low spin:
+3 <
always 4d and 5d
sometimes 3d
high on spectrochemical series
high spin:
+1 and +2
sometimes 3d
low on spectrochemical series
table for free ion and possible electron configurations for octahedral symmetry
Free-ion
High spin
No choice
Low spin
d1
t2g1
d2
t2g2
d3
t2g3
d4
t2g3 eg1
t2g4
d5
t2g3 eg2
t2g5
d6
t2g4 eg2
t2g6
d7
t2g5 eg2
t2g6 eg1
d8
t2g6 eg2
d9
t2g6 eg3
d10
t2g6 eg4
what is paramagnetic
1 or more unpaired electrons
what is diamagnetic
no unpaired electrons
for isolated ion, how do you calculate magnetic moment
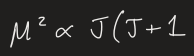
J = S + L
S = spin
L = orbit
for light elements, L doesn't matter so J = S
for 3d metal complexes:
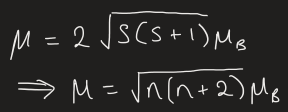
n = number of unpaired electrons
µB = Bohr magneton
equation for crystal field stabilisation energy
for t2gnegm:
energy = n(-2/5 ∆o) + m(3/5 ∆o) + pairing energy
what is inert
reacts very slowly, not practical for lab work
what is labile
reacts very quickly
what are the values that tell you whether its inert or labile
CFSE < -1 ∆o = inert
CFSE > -1 ∆o = labile
what complexes tend to be inert
low spin
for tetrahedral complexes, what orbitals are what labels
what is the difference between the ∆ for tetrahedral vs octahedral with the same ligands
∆Td = 4/9∆o so ligands are split less
what type of spin are tetrahedral complexes
always high spin
what is the Jahn-Teller effect
Jahn-Teller theorem = all non linear nuclear configurations are unstable for an orbitally degenerate state
aka. orbitally degenerate non-linear molecules distort to remove the degeneracy and hence achieve lower energy
unit cell definition
smallest parallel-sided unit from which a crystal can be built purely translational displacements (no rotation)
what is the lattice
the ti-dimensional array of points as obtained by repeating the unit cell
draw a simple cubic cell and calculate how many atoms are in the cell
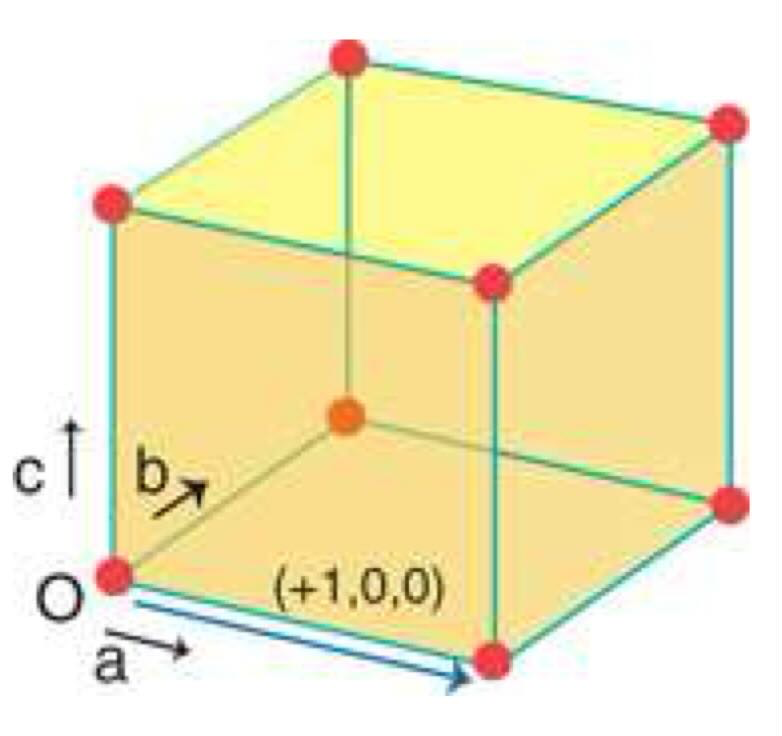
8 × 1/8 = 1 atom per cell
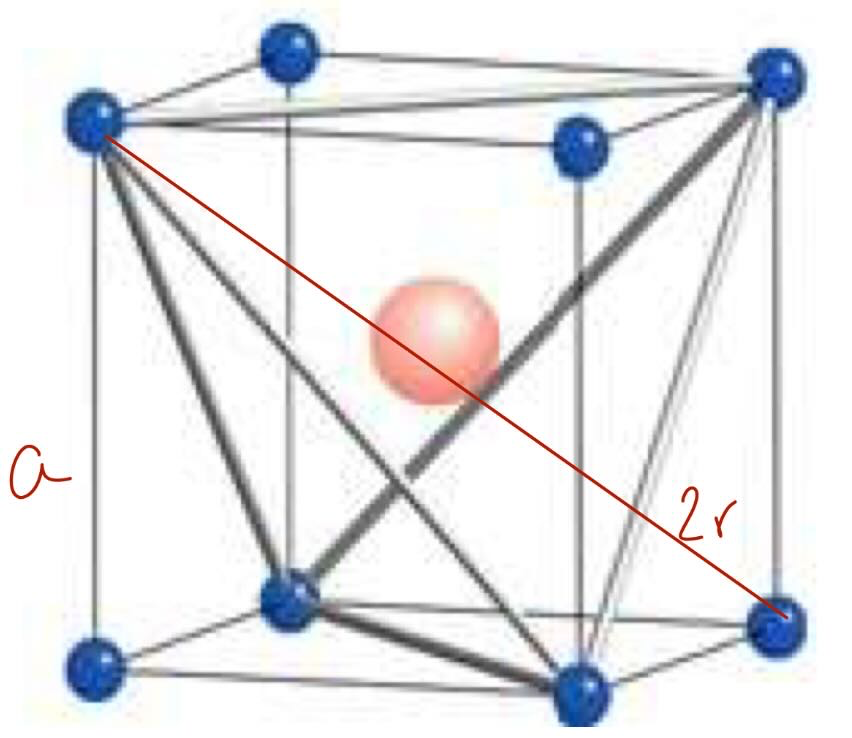
draw a body centred cubic cell and calculate how many atoms are in the cell
1 + 8 × 1/8 = 2 atoms per cell
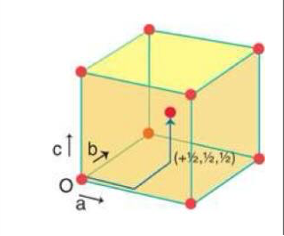
draw a face centred cubic cell and calculate how many atoms are in the cell
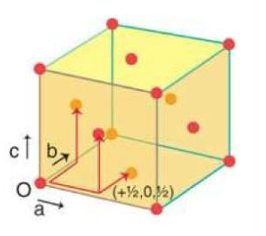
½ x 6 + 1/8 × 8 = 4 atoms per cell

draw projections for the following two structures

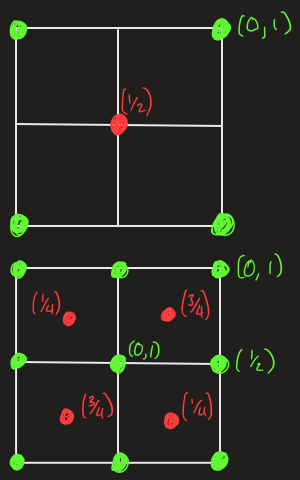
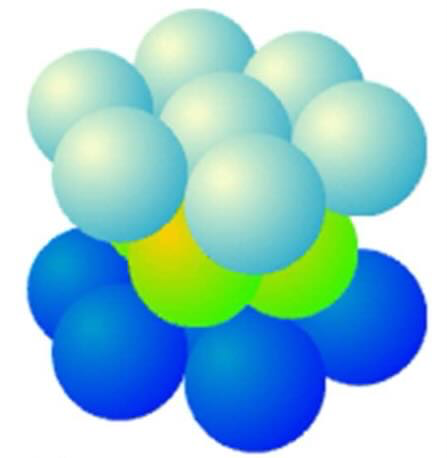
draw hexagonal packing of atoms
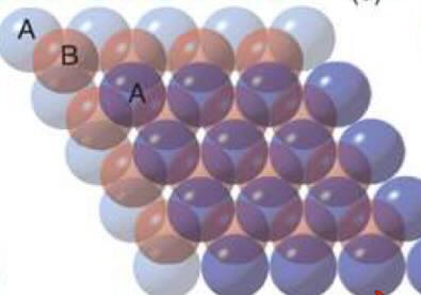
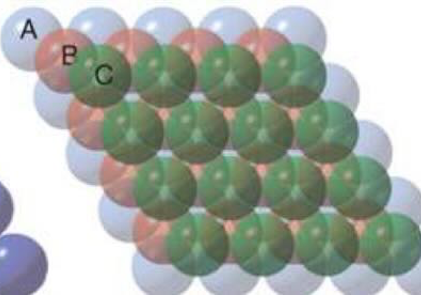
draw face centred cubic packing of atoms
draw simple cubic packing of atoms
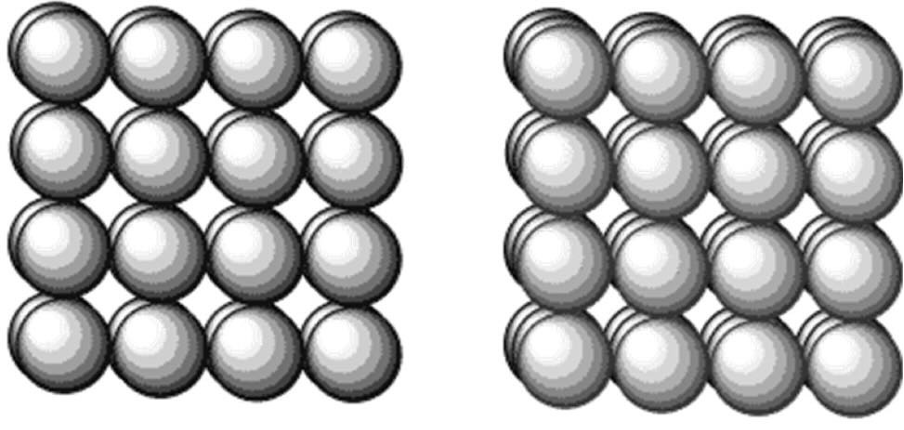
this is non-close packing

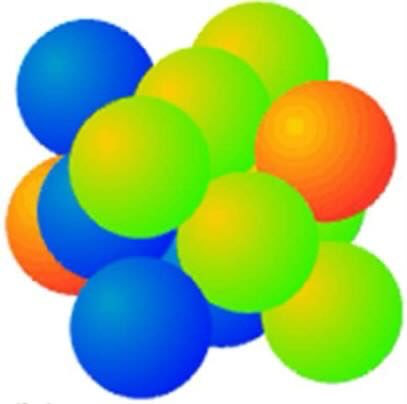
draw body centred cubic packing of atoms
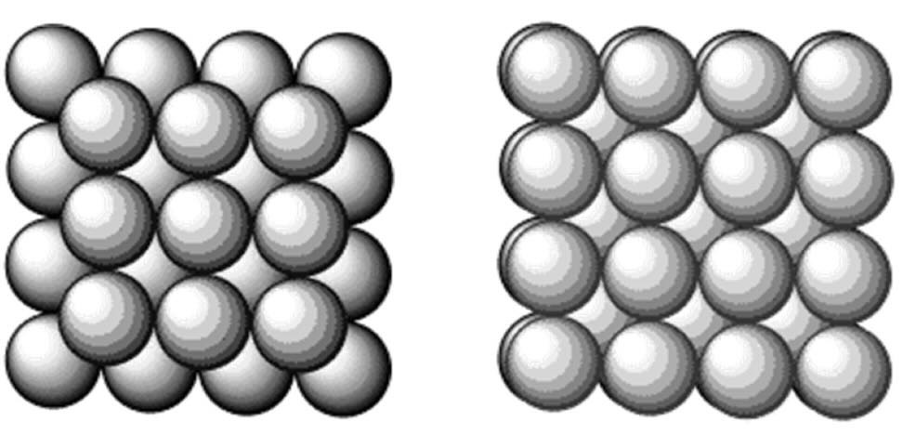
this is non-close packing of atoms
what is the atomic packing factor
fraction volume of the cube occupied by spheres
density of metals equation
density = (number of atoms in unit cell x molar mass) / (NA x unit cell volume (cm3))
1Å = 10-10 m = 100pm = 1 × 10-8 cm
what is an octahedral hole
lies between two triangles of spheres on adjoining layers
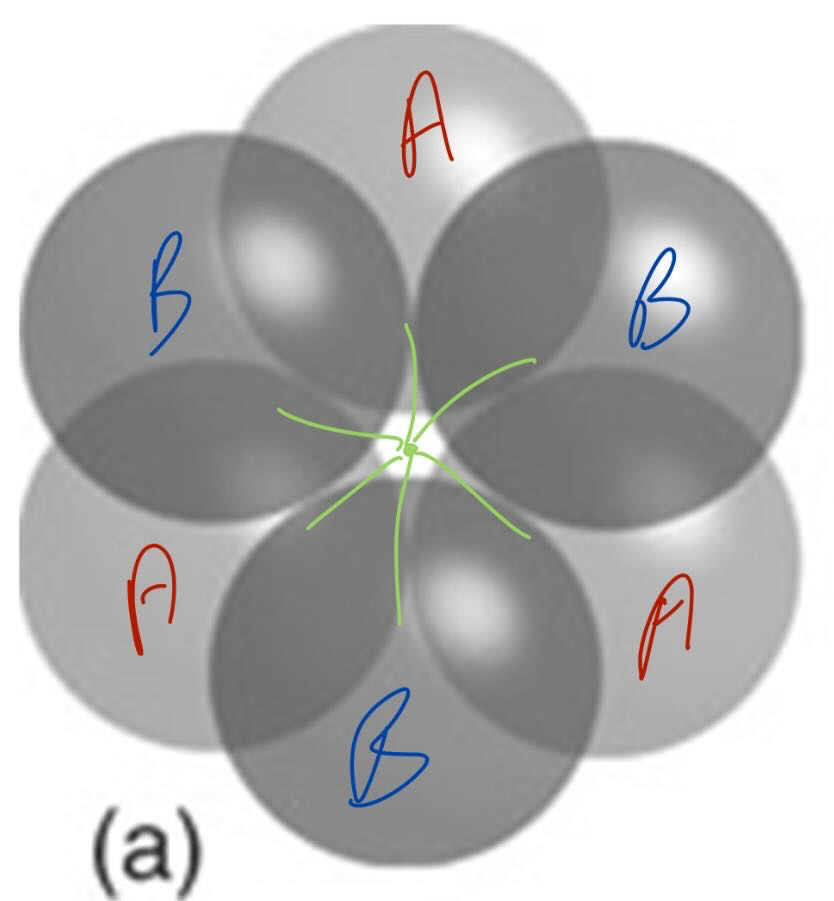
what is the size of an octahedral hole

what is a tetrahedral hole
lies between a triangle of spheres capped by a single sphere
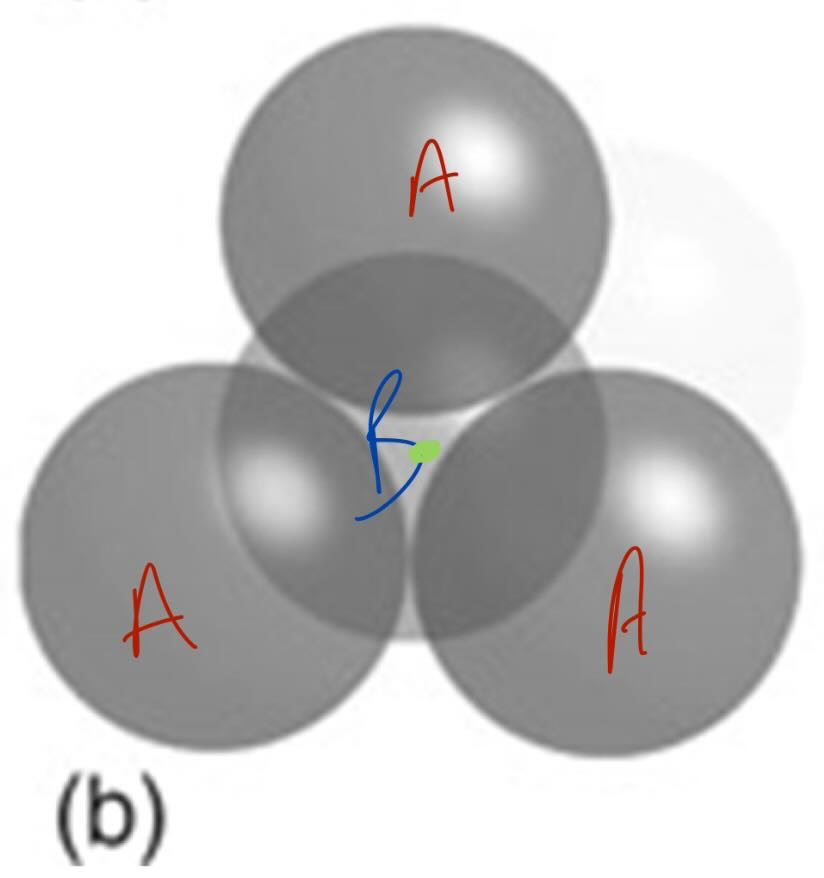
size of a tetrahedral hole
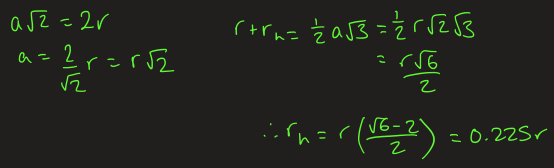
what is a rock salt and when is it stable
face centre cubic arrangement with all the octahedral occupied by counter ions
Oh hole = 41.4% of atom
rock salt is stable when ratio is > 41.4%
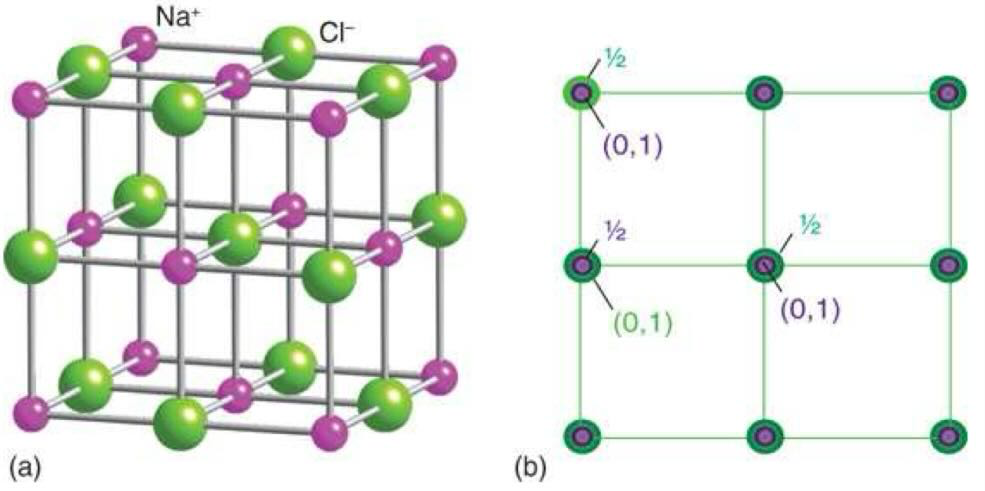
what is zinc blende (sphalerite) and when is it stable
face centre cubic arrangement of S2- and Zn2+ occupying half of the Td holes
Td holes = 22.5% of the atom
stable when ratio between cation and anion > 22.5%
diagonal length = a√3
distance between ions = (a√3) / 4
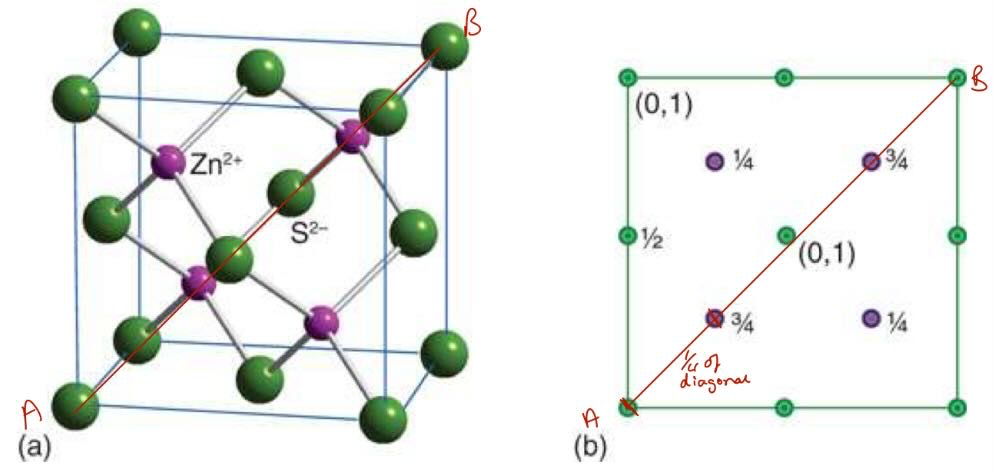
what is the CaF2 (fluorite) structure and when is it stable
face centre cubic arrangement of Ca2+ ions with F- ions occupying all Td holes
ratio of ions > 22.5%
ratio is 1:2 ratio of cations to anions
2:1 ratio is anti-fluorite
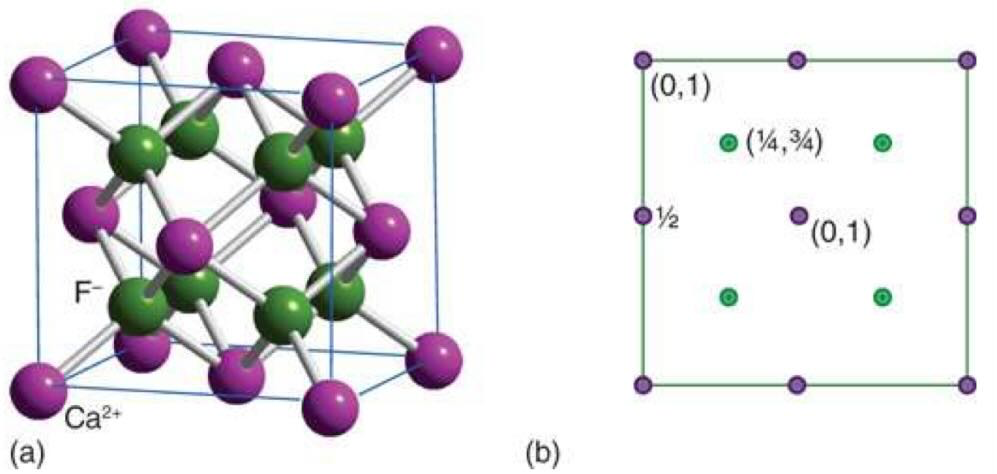
what is CsCl structure and when is it stable
simple cubic arrangement of ions with a counter ion in the middle
need 1:1 ratio of cations : anions
radius is > 73% so very close in size
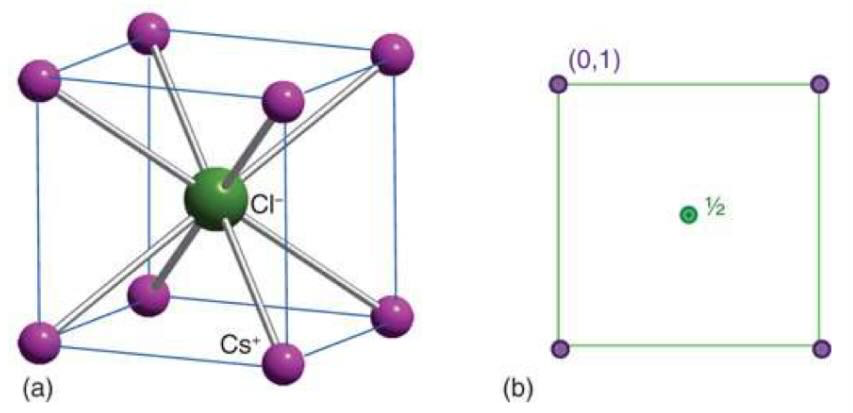
what is the equation for radius ratio
𝛄 = rsmall / rlarge
tells you which structure it will be
what is the equation for Coulombs law
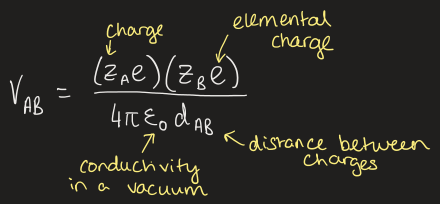
what is the adapted version of Coulombs law to fit crystal structure
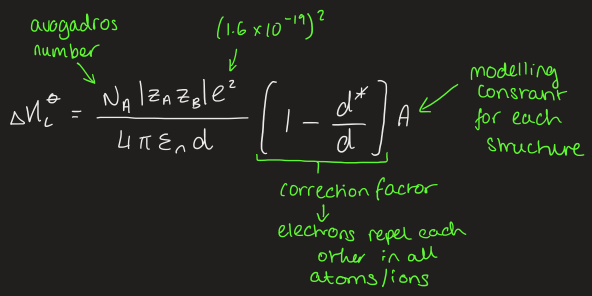

what affects solubility of salts
salts with ions of very different sizes are most soluble
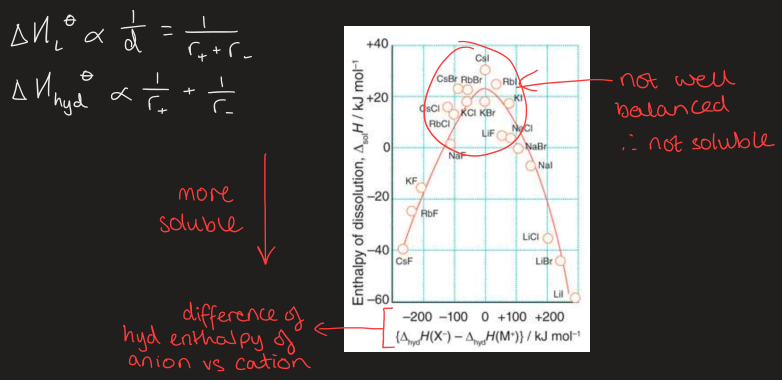
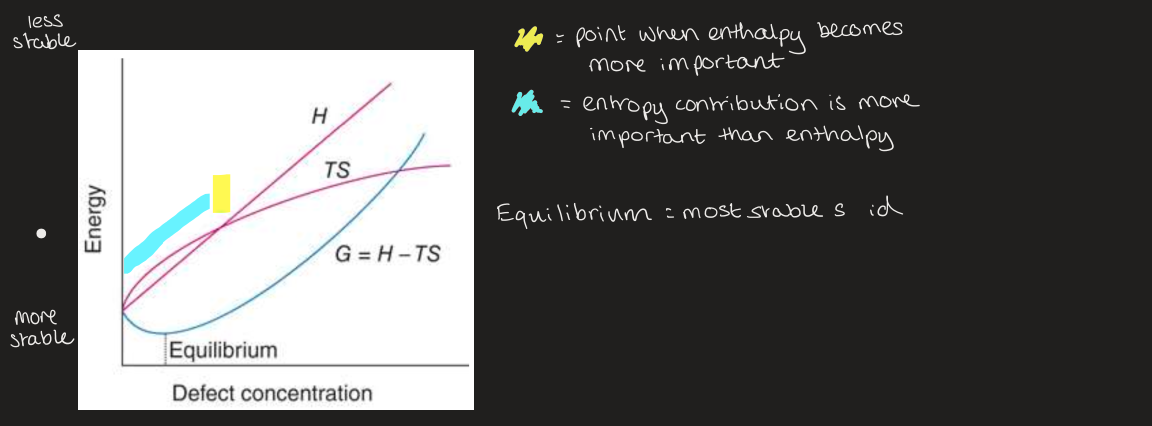
how do energetics change with defect concentration
enthalpy increases with defect concentration
entropy increases but not linearly
increasing T = wants more defects
how does atomic size vary across the periodic table and why
smallest atom at end of period
largest atom at beginning of period
across periodic table there is an increase in effective nuclear charge (Zeff)
down the periods, size increases as Zeff decreases due to shielding
what is the lanthanide contraction
extra protons after the lanthanides contract the atom, causing higher Zeff and therefore smaller radius
how to calculate ionic size
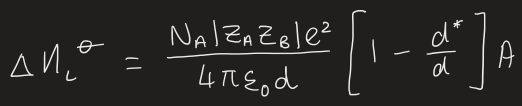
measure lattice enthalpy then unknown radius can be estimated by determining d and using radius of second ion
how does the characteristics change with hard/soft ions interacting
Two hard ions
Highly ionic
∆HL-calc = ∆HL-exp
Hard ion + soft ion
Hard ion = polarsive
Soft ion = polarisable
∆LHexp > ∆LHcalc
Covalent
Two soft ions
Both polarisable
Large orbital overlap
Large covalency
∆LHexp >> ∆LHcalc
what happens in a metathesis reaction
molecules will swap partners in order to create molecules with 2 soft elements and 2 hard elements, rather than one of each
what are the key points in terms of general trends of the periodic table
trends in radii depend on Zeff
trends aren’t smooth
ionic radii aren’t fixed due to partial covalencey
hard/soft model can predict which ions partner
what oxidation states can H be
+1 or -1
what are interstitial hydrides
chemical compounds where hydrogen atoms occupy the spaces (interstitial sites) within the crystal lattice of a metal or alloy
what is oxide, peroxide and superoxides
oxide: O2-
peroxide: O22-
superoxide: O2-
what is the simplification of the Born-Mayer equation to approximate lattice enthalpy
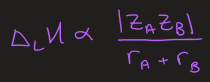
why should peroxides decompose

r(O2-) < r(O22-) so ∆LH of oxide is larger
BUT
if r(M+) is large then ∆LH is always small so less driving force for decomposition of peroxides
large cations stabilise large anion so:
Na2O2 = stable
Li2O2 = unknown (unstable)
what ligands bind to alkali metal cations
crown and cryptand ligands
18-crown-6
Li+ < Na+, Cs+ < Rb+ << K+
15-crown-5
Cs+ < Rb+ < K+, Li+ << Na+
12-crown-4
Cs+ < Rb+ < K+< Na+ << Li+
do group 1 metals show tendency for covalency and give any examples
only Li
smallest cation so largest charge density and polarising
CH3Li
why do group 2 carbonates take a lot of energy to decompose
large cation stabilising large anion
decomposition releases CO2 so S is favourable
decomposition of CO32- to O2- gives large gain of ∆HL for small M2+
is covalency common in group 2 compounds
common in Be compounds and sometimes Mg due to Be2+ having high charge density and Grignard reagents containing Mg
what is the anomalous chemistry of Be
covalency
amphoteric compounds
coordination number can vary
can be tetrahedral or octahedral
normally small size = low coordination number
why is Gallium different to other metal structures in group 3
short Ga-Ga distances (2.44Å) and Ga—Ga distances (2.75Å)
4s2 electrons are delocalised and 4p1 electrons are held in the bonds
what is the reason for difference in ionisation energies for group 3 elements
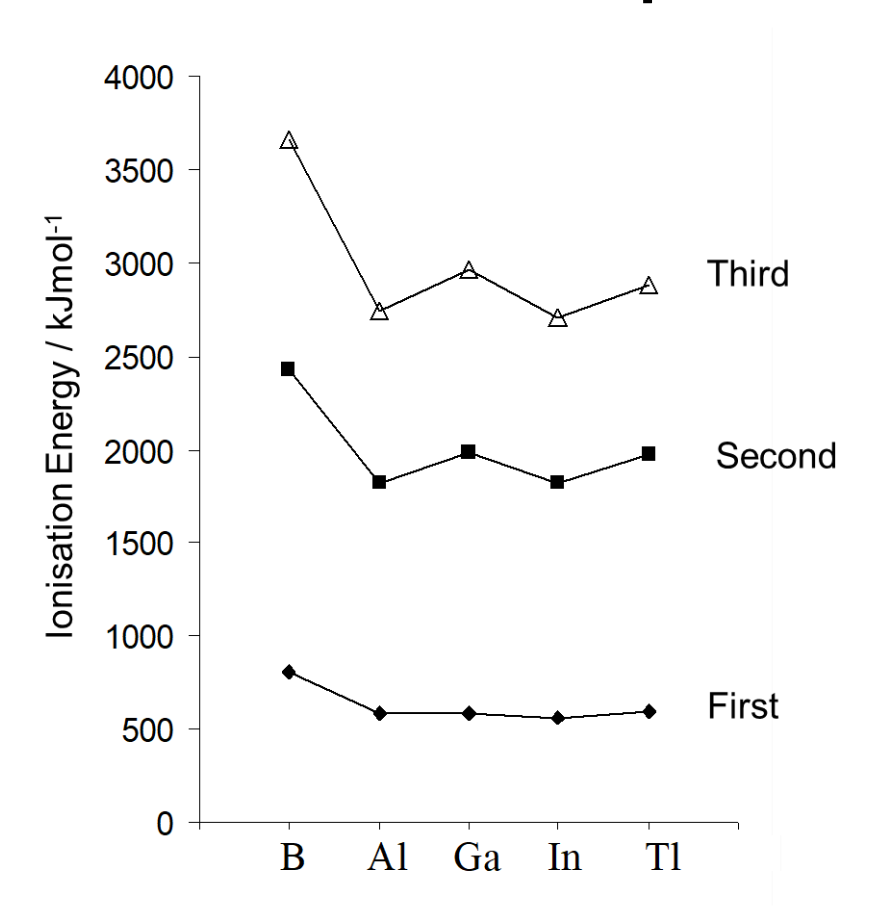
B and Al don’t have core d-electrons, where as Ga does
Ga has much higher Zeff
Ga also has inert pair effect
Tl has even higher ionisation energy due to greater Zeff
f electrons in core
how does stability of M3+ ions decrease as you go down the group
becomes less stable
Al3+ = very stable
Ga3+ = less stable
Tl3+ = very unstable
easier to reduce as you go down the group
what are the possible halides of group 3 metals and why
M(+1)
possible for all except Al
Al3+ forms strong bonds so is always favourable to Al+
GaX, InX for X = Cl, Br, I
TlX works for all halides
M(+2)
mixed valent
not possible for Al
M(+3)
form compounds with all halides (F, Cl, Br, I)
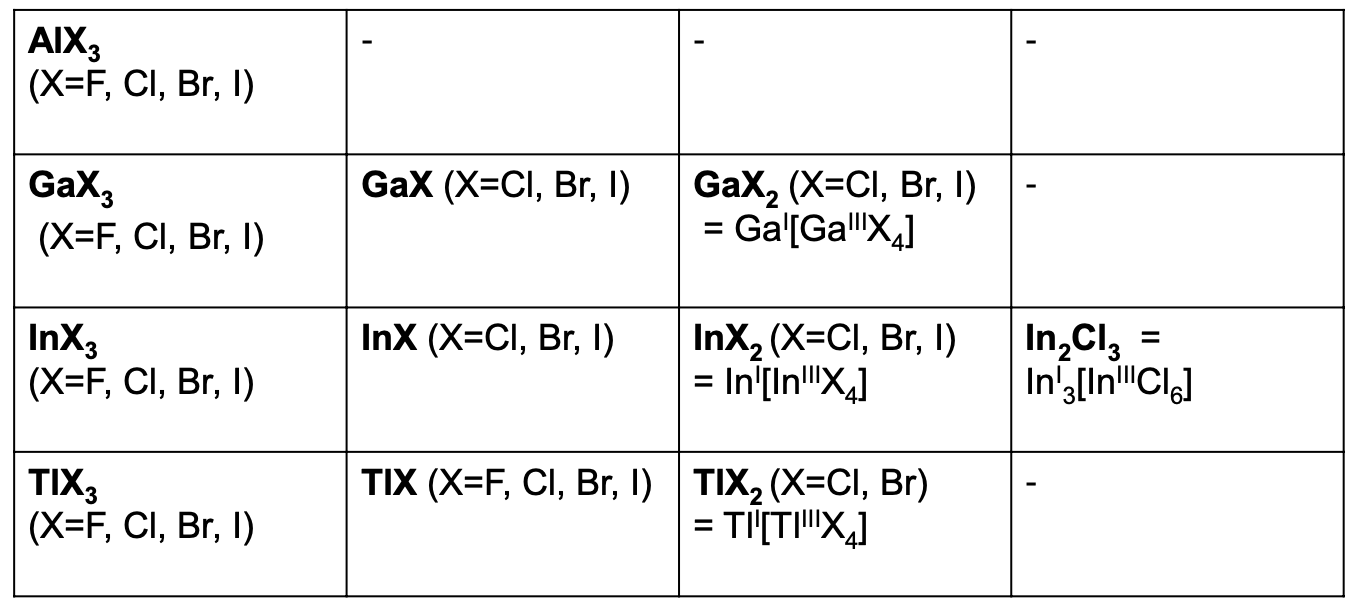
what is a mixed valent compound and why do they occur
mixture of oxidation states as odd electrons are very reactive so compounds want to avoid odd electron compounds
what are the different structures of boron hydrides
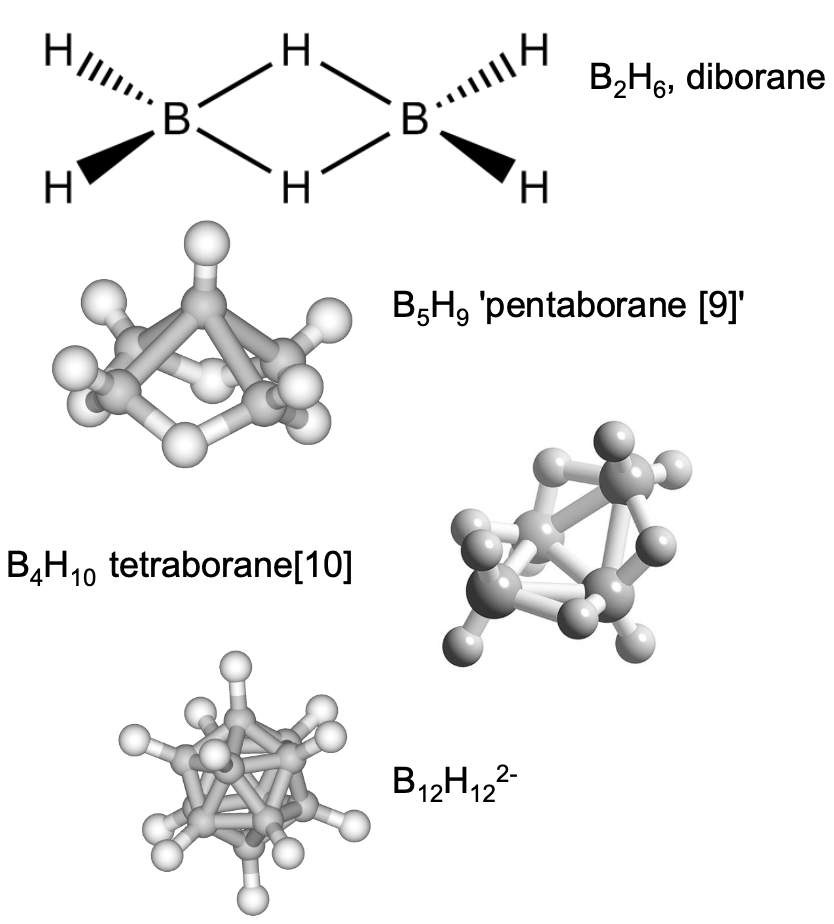
describe the bonding in B2H6
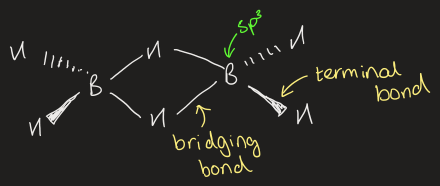
B-H (terminal) = 1.19 A
stronger
B-H (bridging) = 1.31 A
weaker
8 total bonds between 12e- so doesn’t fit Lewis diagram
multi centre bonds
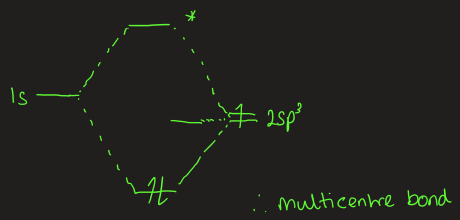
key points for group 3
s & p valence orbitals differ in energy
ns2 pair of electrons is tightly bound, especially after TM
+3 O.S. is more difficult to reach for Ga, In, Tl, where bonds are weaker
inert pair effect
boron forms e- deficient covalent molecules with multi centre bonds, where bond order < 1
what are the allotropes of carbon and their structure
diamond
tetrahedral + giant
sp3 hybridised
C60 : buckminsterfullerene
sp3/sp2 hybridised
molecular
graphite/graphene
sp2 hybridised
graphene is a layer of graphite
intermolecular forces between layers
nanotubes
rolled graphene
properties of group 4 elements
C, Si, Ge = giant covalent structures
strong σ bonds due to large separation between σ and σ* for carbon but smaller for Si and Ge
for Si and Ge, excitation of e- allows them to carry charge
C = low conductivity
Si, Ge = semiconductor
Sn(white) and Pb are metallic lattices
weak covalent bonds
lower ionisation energies
high conductivity
what are ionic carbides
negative O.S. for C
e.g. CaC2, Na2C2 contains [C=C]2-
lattice enthalpy stabilises reactive anion
e.g. MgC3 contains [C=C=C]2- has average O.S. of -2/3
what are the formulas for the structures of group 4 hydrides
C:
CnH2n+2
CnH2n
CnH2n-2
most stable of the group
Si:
SinH2n+2
pi bonds for Si are very weak
large atoms = poor orbital overlap
only stabilised with bulky substituents
Ge:
GenH2n+2 (n < 9)
SnH4
difference in bond strengths between C and Si
larger atoms of Si = weaker bonds
for more electronegative elements, stronger bonds form with Si as ∆χ is larger so more polar bonds
weaker Si-H bonds will readily react to form stronger Si-O bonds, whereas C-H bonds are stable enough to not react as easily
what are the types of oxides of carbon and why do they form
CO, CO2, C3O2
CO and CO2 are gases under ambient conditions
C3O2 polymerises at room temp
other compositions possible but all highly reactive
pi bonds form due to small size of C
what are the oxides formed with silicon and germanium
no pi bonding
SiO2
alpha-quartz
giant covalent solid made of single σ bonds
each silicon is bonded to 4 oxygens and oxygen to 2 silicons
polysilicates
minerals where charge is balanced by cations
[SiO3]n2n-
[Si4O11]n6n-
what are the oxides of tin and lead
SnO2 and PbO2
coordination number = 6
since they are large elements, can have a high coordination number
fit more atoms around them
both at O.S. of +4
each oxygen corner shared by 3M
SnO and PbO
+2 O.S. (inert pair)
layered structure with stereochemically active lone pair
Pb3O4
mixed valent compound
average O.S. of +8/3
key points about group 4
progression of non-metal → semi-conductor → metal
negative O.S. possible
strong pi bonds for C as it is small
inert pair effect so can have +2 O.S. for Ge, Sn, Pb
large size = high coordination number
properties of group 15 elements
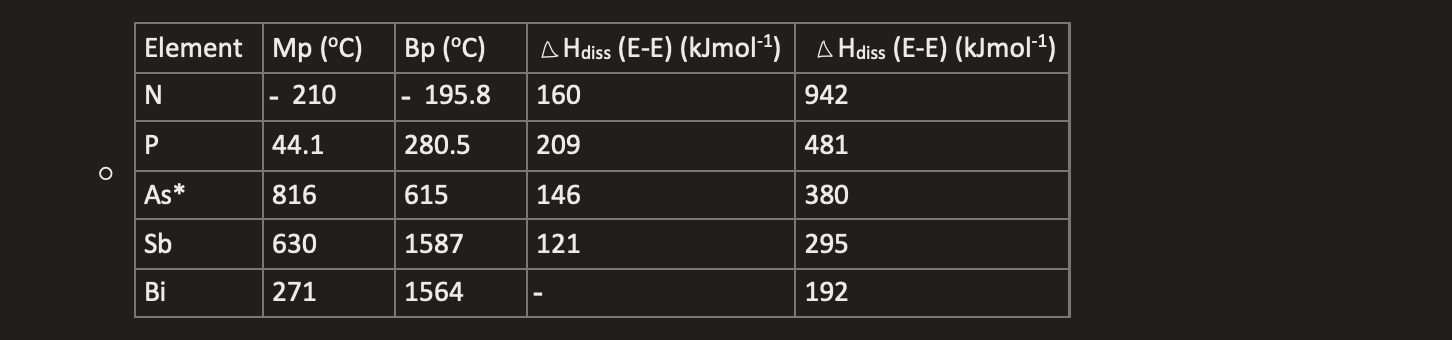
only N is a gas at room temp
N-N has lone pair repulsion for single σ bond
E(N≋N) » E(N-N) due to good pi overlap on small atoms
E(P≋P) < 3 x E(P-P) due to weak pi bonding on larger atom
what are the allotropes of phosphorus
white phosphorus
molecular P4 units
significantly more stable than P2
red phosphorus
chain structure of opened up tetrahedron
5 different crystalline forms and glassy forms
all react with oxygen (explosively) as P-O is very favourable
nitrides, phosphides and arsenides
N3- (nitride) and N3- (azide)
phosphides contain P-P bonding like elemental forms
arsenides contain As-As and As-M bonds
what are the halides of group 15 and what is observes when bonded to 5 chlorines
NF5 cannot exist as N is too small to fit 5 x F around it
Can have N(2+) and P(2+)
issue of odd electrons is avoided as they are paired in the bond
trihalides exist with the inert pair effect
ns2 becomes more tightly bound down the group
Bi-X bonds are too weak to compensate for removing ns2 pair (except F)
observations for XCl5
NCl5 = not stable as N is too small
PCl5 = stable as a gas
AsCl5 = unstable above -50oC due to inert pair effect
SbCl5 = stable at room temp as 5s2 is less tightly bound
BiCl5 = not known as 6s2 is very tightly bound
what is the structure of SbF5
tetrameric Sb4F20 molecules
coordination number of 6
similar for solid As and Bi halides
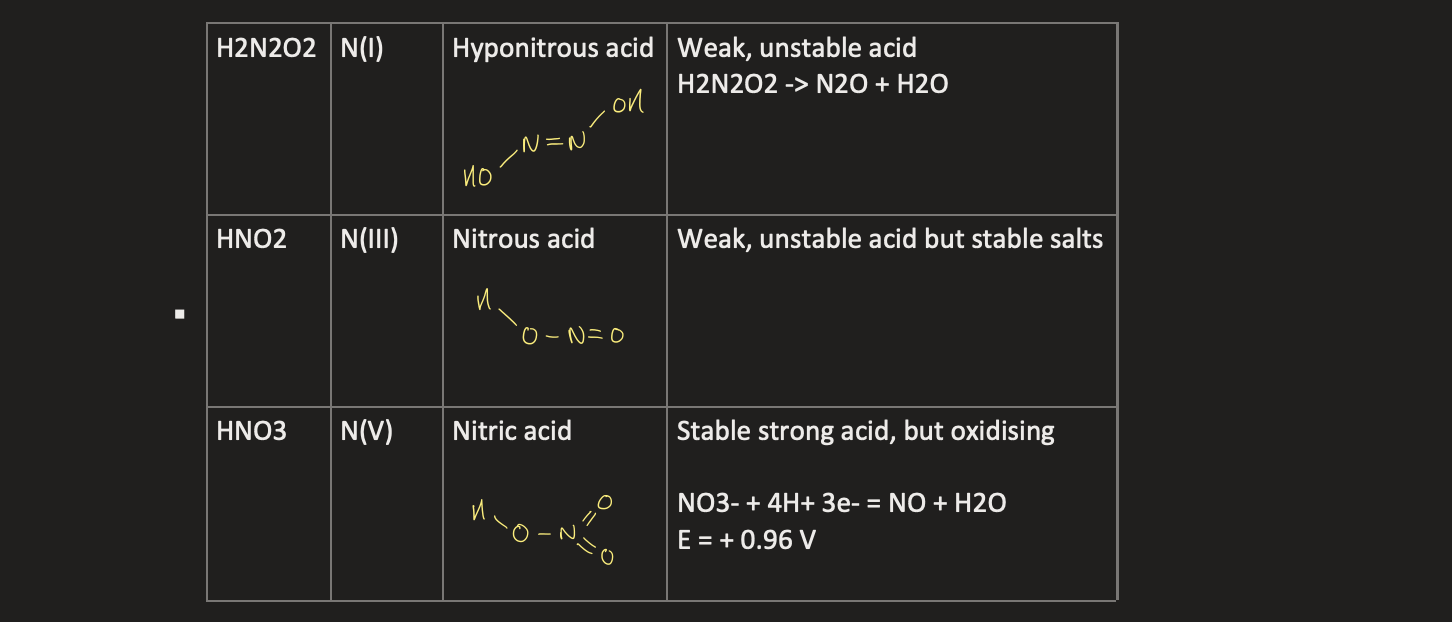
what are the different oxyacids for nitrogen and phosphorus
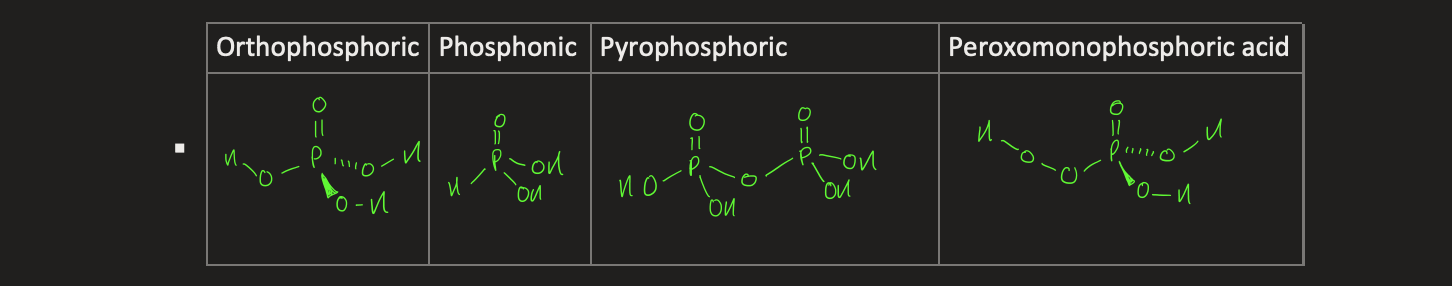
what is Paulings rule
-XOp(OH)q
X = P, N, S, Cl
X=O is an oxy group
X-OH is hydroxy group
XOp(OH)q + H2O → [XOp(H2O)q-1O]- + H3O+
pKa = 8 - 5p
what happens as you remove protons from tribasic acids and why
pKa becomes larger so become weaker
charge on anion is becoming more negative and it becomes harder to remove a proton from an already negatively charged ion
key points for group 15
N has strong pi-bonds and low coordination number due to small size
P has σ bonds usually but can make P=O pi bonds as O is small
+5 O.S, can be found for N but not NF5
+5 O.S. very unstable for As and Bi due to inert pair effect
Paulings rule is used to predict pKa of oxyacids
properties and states of group 16 elements
electron affinities:
adding electrons becomes more favourable on RHS of periodic table
EA1 very favourable
EA2 endothermic but ∆LH favourable so still happens
O can be O2 or O3 gases
all other elements are solids
what bonding does O prefer vs S
E(O=O) > 2 x E(O-O)
repulsion between lone pairs
strong pi bonds, weak σ bonds
E(S=S) < 2 x E(S-S)
better to make 2 single bonds
larger atom so weak pi bonds
what are the different allotropes of sulphur
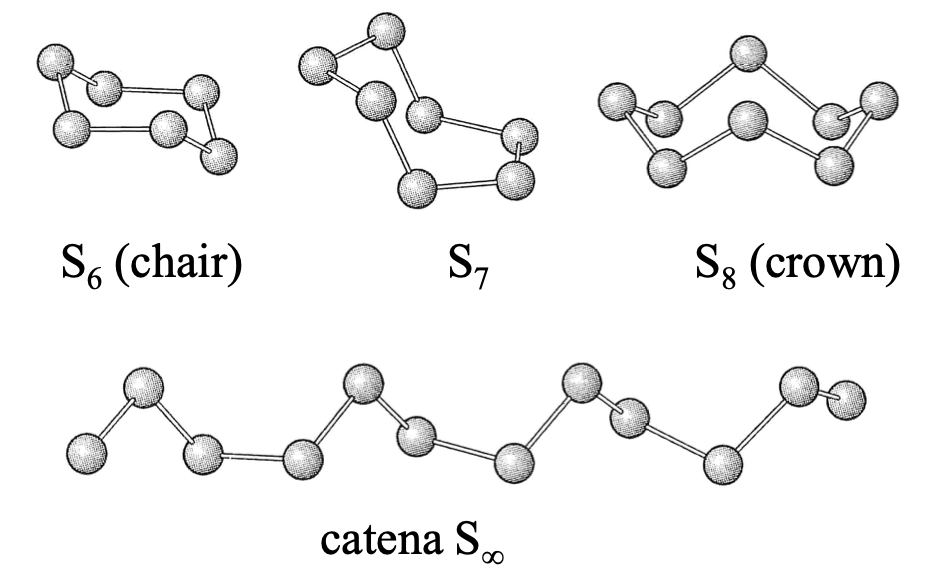
more allotropes than any other element
all have S-S
S8 most stable
what are the structures of selenium and tellurium (group 16)
Se:
many allotropes
Te:
moving towards metallic sturcture
atoms from different chains very close together
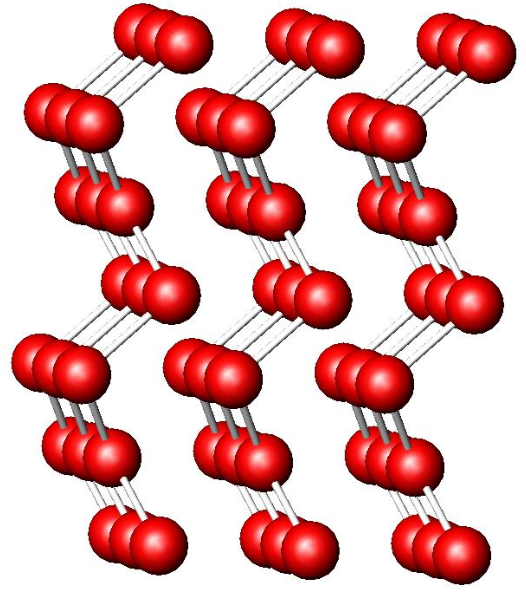
what are the different types of ionic oxides, and which are more likely to decompose
oxide - MgO
peroxide - Na2O2
superoxide - KO2
peroxides and superoxides are thermally unstable and produce O2 (g), which is entropically favourable
give examples of molecular and giant covalent oxides
molecular:
N2O
CO
CO2
P4O10
giant covalent:
SiO2
B2O3
how do sulphides structure themselves
layered structures with close S—S contacts (van Der Waals)
disulfides and polysulfides exist
S-S single bonds are relatively stable so disulfides/polysulfides like peroxides
what oxygen halides are in positive oxidation
OF2 and O2F2 as F is more electronegative
O2F2 dissociates into OF2 and F radicals
halogen oxide examples
Cl2O
ClO2
Cl2O6
Cl2O7
Br2O
BrO2
I2O
I2O5
what are the rules for halogen sulfides
Even O.S. = even number of electrons
SF2, SCl2, SF4, SCl4, SF6
Odd O.S. occurs when odd electrons are paired in bonds because of the 2 S atoms
S2F2, S2Cl2, S2Br2, S2I2, S2F10
can also be less then +1 O.S.
SnCl2, SnBr2 (n = 1-8)
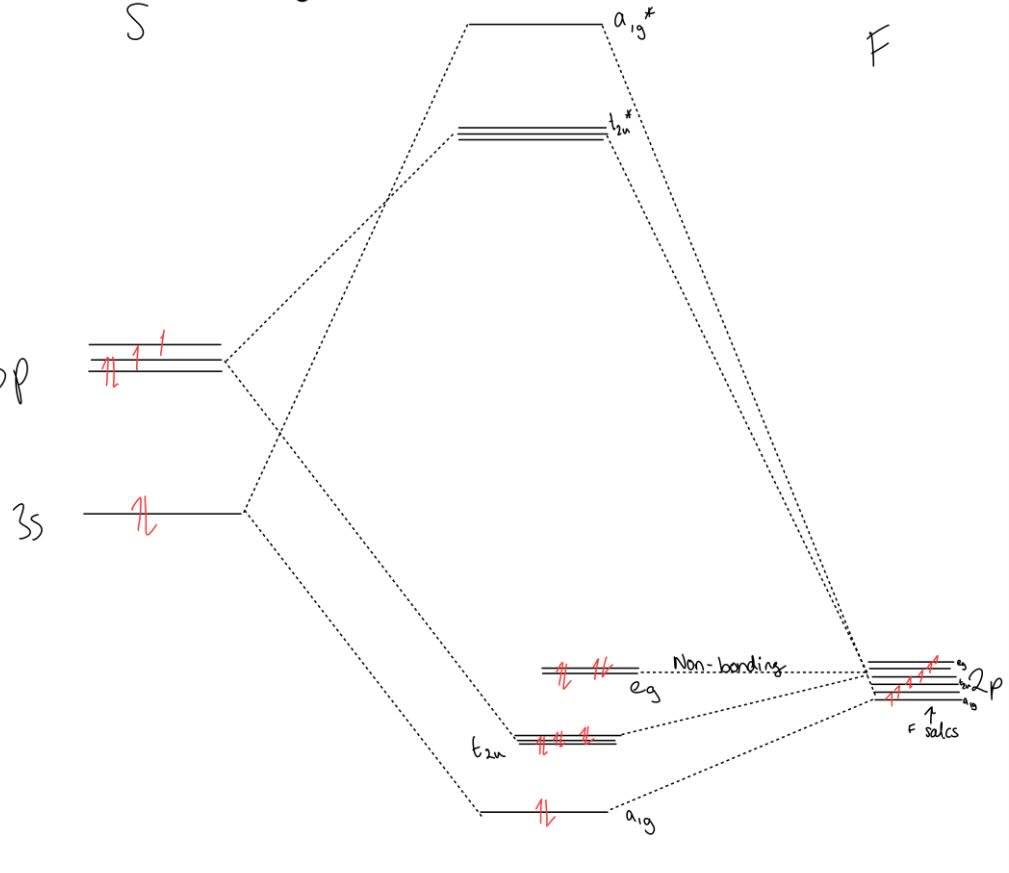
what is the MOELD of SF6 and what is the bond order/why does it work
Bond order = 2/3
Only works for S as O is too small to fit 6 x F around it (would be too sterically crowded)
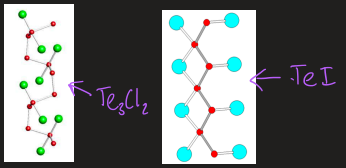
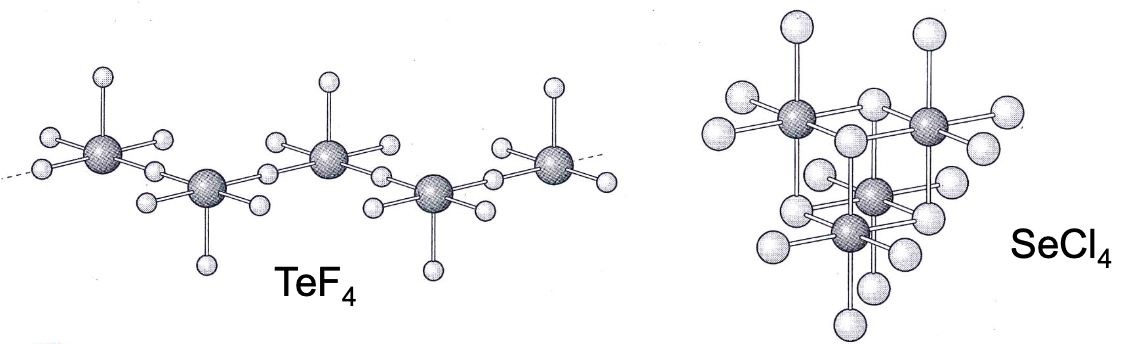
what are the halides of selenium and tellurium
TeF4 and SeCl4 have coordination numbers of 5 and 6 due to large size
bridging halides
partial halogenation to retain chains if Te reacts with small amounts of halogen