Covalent Model
1/54
Earn XP
Description and Tags
Thus far: S2.2 → covalent model & Intermolecular forces
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
55 Terms
Covalent bond
the bond between atoms as a result of sharing (pairs of) electrons
the atoms do this to gain stability
bond order (strength and type of covalent bond)
STRENGTH: single < double < triple
LENGTH: single > double > triple
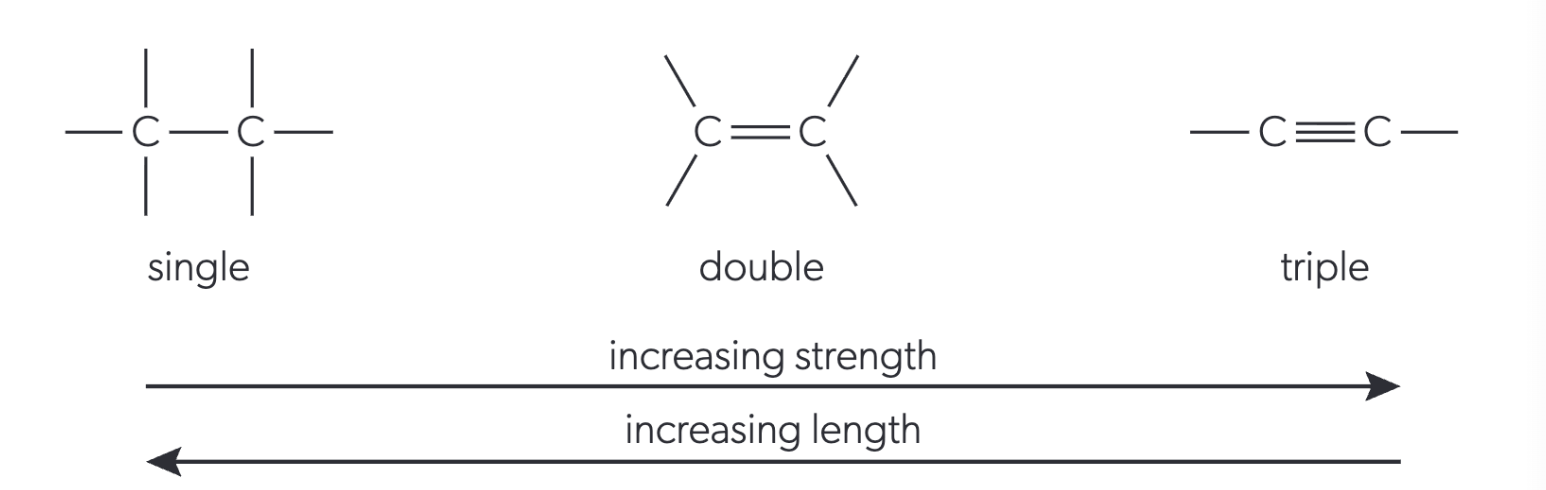
relationship between bond strength, length and bond enthalpy
⬇ bond length = ⬆ strength = ⬆ bond enthalpy
coordination/dative bond
when both electrons in a covalent bond originate from a single atom
*Notation of an arrow in lewis structure (ex. CO)

octet rule
the tendency of atoms to gain electrons to have a valence shell of 8 electrons
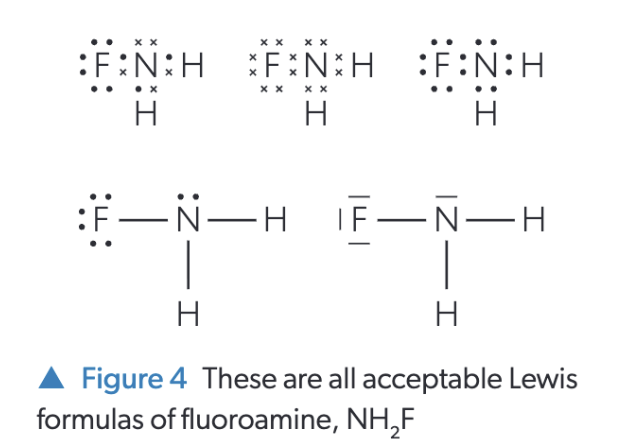
how to draw lewis structure
ID valence electrons of each atom (periodic table group)
H is almost always a terminal
H cannot be the central atom of a molecule why?
Hydrogen only has 1 (valence) electron (to share) thus H is a terminal atom
lewis structure of CO2
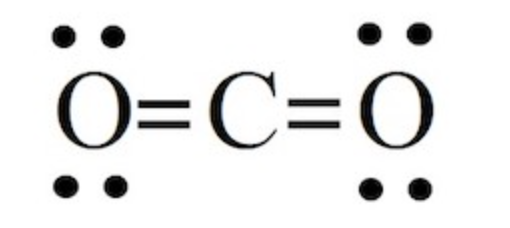
why is carbon special in its covalent bonding
Carbon always shares it’s 4 electrons
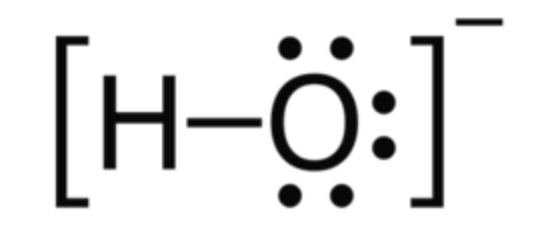
notation of ions in lewis structure
use brackets around the entire molecule and add the charge in the top left
see OH- example
exceptions to the octet rule
Boron & Berylium — when central atoms will be ‘electron deficient’ (will have less than 8 valence ēs)
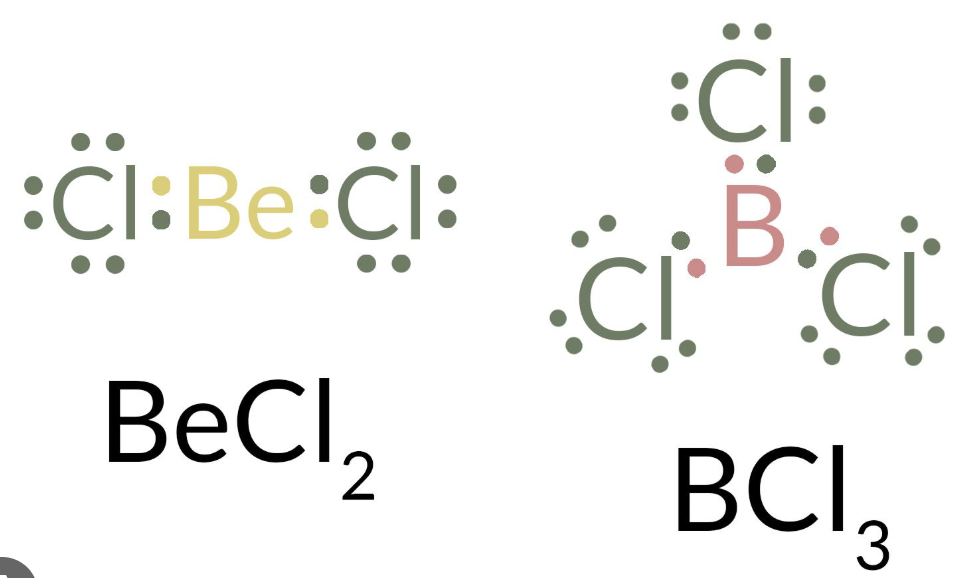
valence shell electron pair repulsion (VSEPR) model
allowing for the prediction of a molecules shape & geometry based on the repulsion of the atoms electron (pairs)
electron domain
regions of high electron density due to a “lone pair” of electrons
electron domain geometry
the shape the electron domains form
molecular geometry
the shape formed by (just) the atoms of a molecule
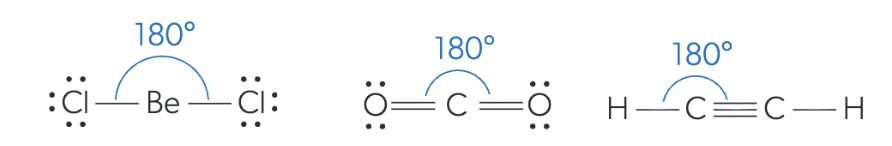
electron & molecular geometry + bond angle of 2 electron domains
linear electron & molecular geometry
bond angle: 180°
the 2 electron domains repel each other
electron geometry & bond angle of 3 domains
trigonal planar electron geometry
bond angle: 120°
molecule geometry of 3 electron domains
if 1/3 of the domains is a lone pair then the molec geo becomes ‘V-shaped’
if 3/3 of the domains are bonding domains then the molec geo is trigonal planar (like the ē geo)
bond angle of 3 electron domain (if there is a one lone pair)
if there is a lone pair as one of the electron domains; they will have a larger repulsion, thus the angle will be slightly smaller than 120°
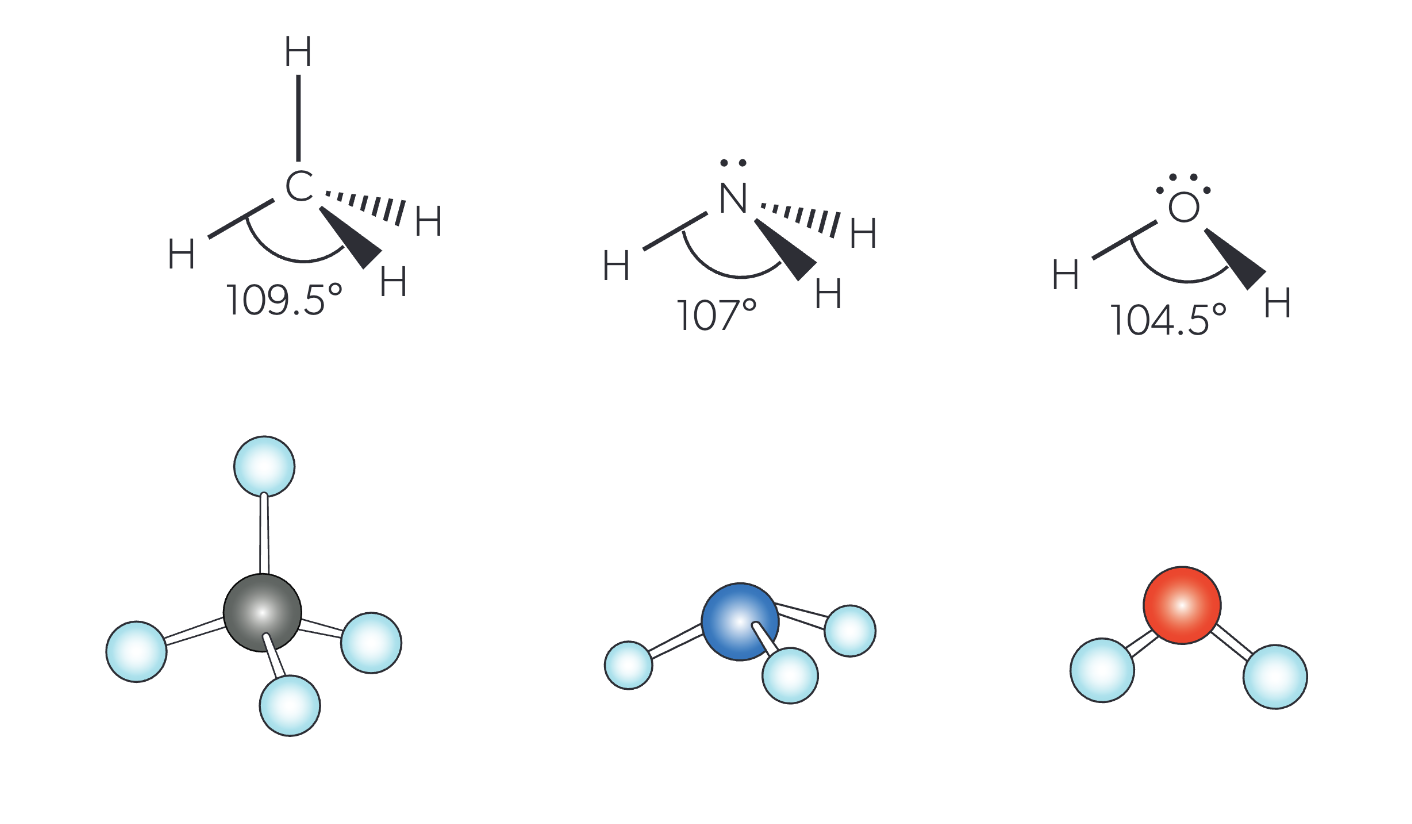
electron geometry & bond angles of 4 electron domain
tetrahedral electron geometry
however the bond angle is dependent on the # lone pairs:
0 lone pairs = 109.5°
1 lone pair = 107°
2 lone pairs = 104.5°
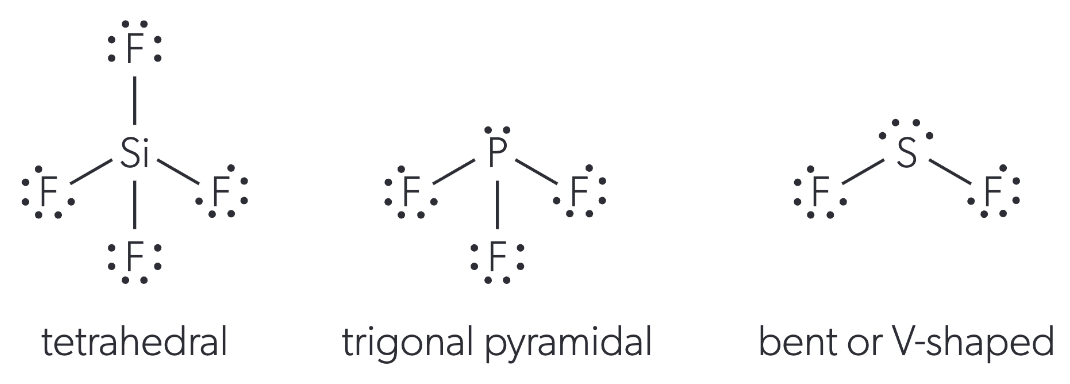
molecular geometry of 4 electron domains
dependent on the # of lone pairs:
0 lone pairs = tetrahedral
1 lone pair = trigonal pyramidal
2 lone pairs = V-shaped
electronegativity
the ability of an atom to attract electrons (the electrons that are shared within a covalent bond) towards itself
explain polarity (of covalent bonding)
polar covalent bonds result from a difference in electronegativity of two bonded atoms
they have a bond dipole (opposing difference in charge)
non polar bonds in relation to electronegativity
non-polar bonds mean there is an equal sharing of electrons within the [covalent] bond
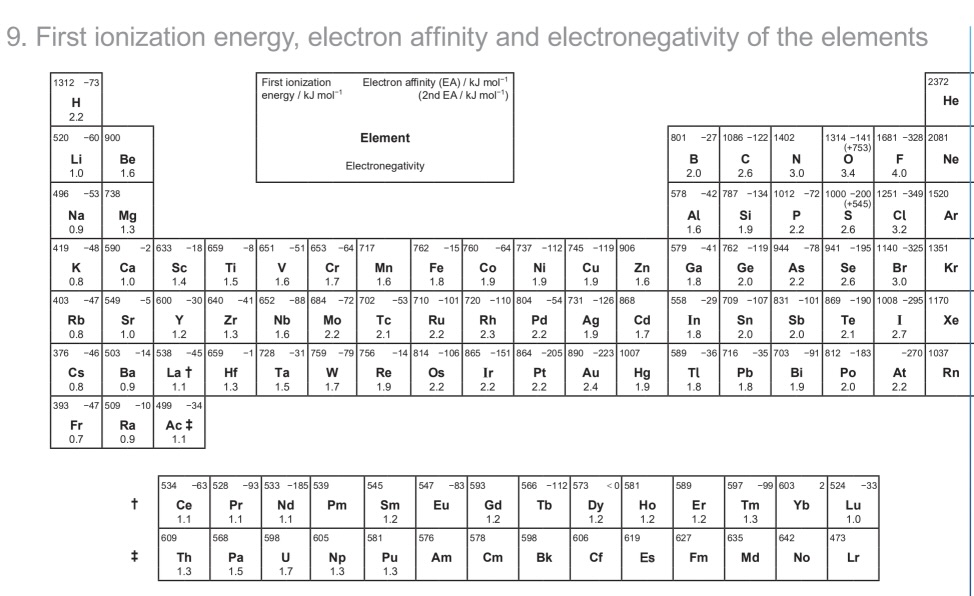
how to identify polarity of bonds from data booklet values
differences of
0.1-0.4 = weakly polar covalent bond
0.5-1.7 = (fully) polar covalent bond
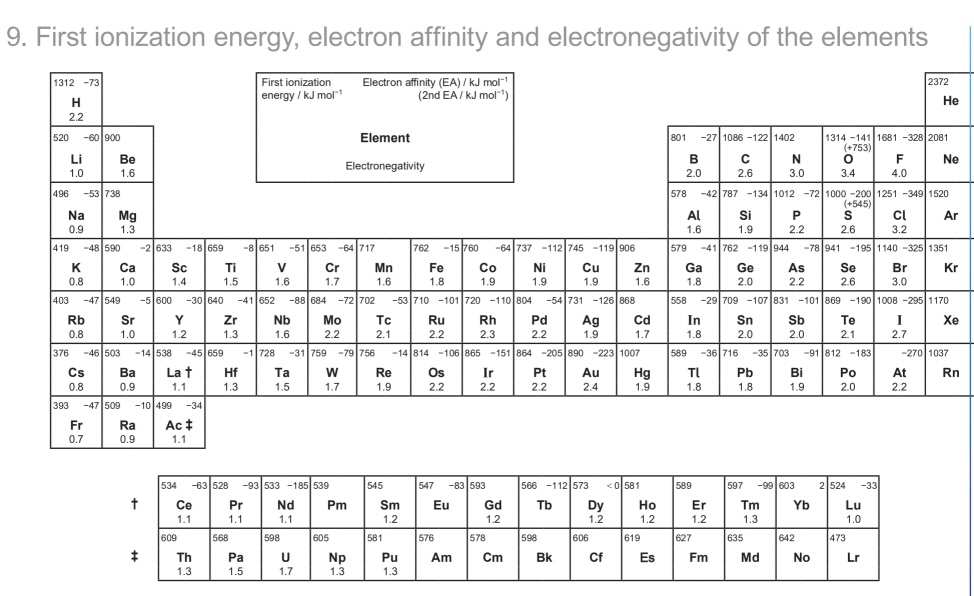
what makes a molecule polar
molecular geometry/net dipole moment
+
bond polarity
dipole
when a molecule has a -ve and a +ve end, between two bonded atoms
net dipole moment
having a -ve end and a +ve end, within a whole molecule
this net value is based on the dipole between each atom of the molecule
thus you are able to “cut” the molecule in half and evidently have a +ve and -ve end
relate net dipole moment to molecular polarity
molecules can only be polar when there is a net dipole moment
TRUE OR FALSE: some molecules can have polar and non-polar regions.
TRUE
ex. large molecules
examples of non-polar molecules
CO2 / carbon dioxide
homonuclear diatomics (i.e. H2 , O2)
CH4
examples of polar molecules
H2O / water
NH3
HCl
H2S
TRUE OR FALSE: If all covalent bonds in a molecule are non-polar, the entire molecule is non-polar.
TRUE
There is no net dipole moment if the terminal atoms of a molecule with the geometry of _________ , ________, or ___________ are all the same.
tetrahedral
linear
trigonal planar
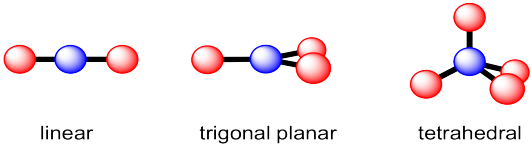
how to signify electronegativity on lewis structure/formula of molecule
note: difference in electronegativity is demonstrated by a difference in length of the arrows
intermolecular forces
forces in-between molecules
a change in intramolecular forces results in…
a chemical change
a change in intermolecular forces
a physical change (change in physical properties such as melting pt and boiling pt)
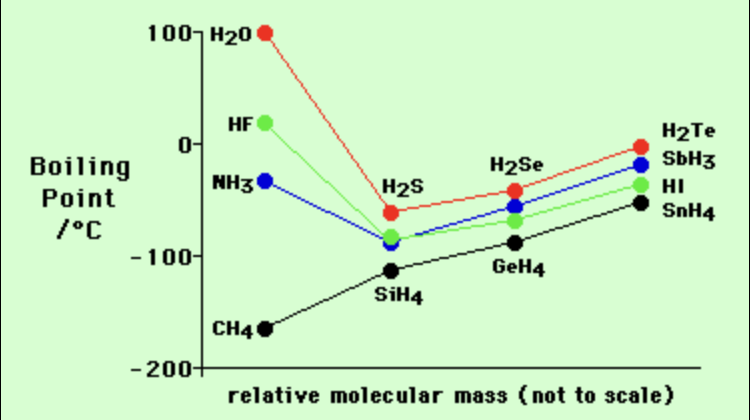
what pattern can be identified about group 14 hydrides, in relation to their boiling point
as you proceed ⬇ the group, the boiling point of the elements hydride ⬆
what intermolecular force applies to all molecules
London dispersion forces (LDF)
london dispersion forces
a weak force caused by the movement of electrons within a molecule (or atom)
this movement of electrons leads to a temporary instant dipole
what is the relationship between molar mass & boiling pt and why
positive correlation:
as boiling pt ⬆ = molar mass ⬆
bc larger molecs have stronger LDFs thus physical properties are affected
explain LDFs using H2 as an example
a pair of electrons will move (slightly) closer to one atom (within the bond)
thus making the H2 molecule now slightly polar (instantaneous dipole)
if another molecule is nearby, the recently moved ēs will repel a pair of ēs of the neighbouring molecule (dipole-induced dipole)
and so on and so forth…
polar molecules can have what types of intermolecular forces
LDFs
dipole-dipole
H bonding
what molecules have dipole-dipole forces
polar molecules aka molecules with a net dipole moment
explain dipole-dipole intermolecular forces
because of the difference in EN between the atoms in the molec, the electrons are ‘pulled’ closer to the more EN atom
what type of intermolecular force is hydrogen bond(ing)
a special dipole-dipole bond
when does H bonding form
when there is a strong dipole involving H
H is bonded with F, O or N —highly EN atoms
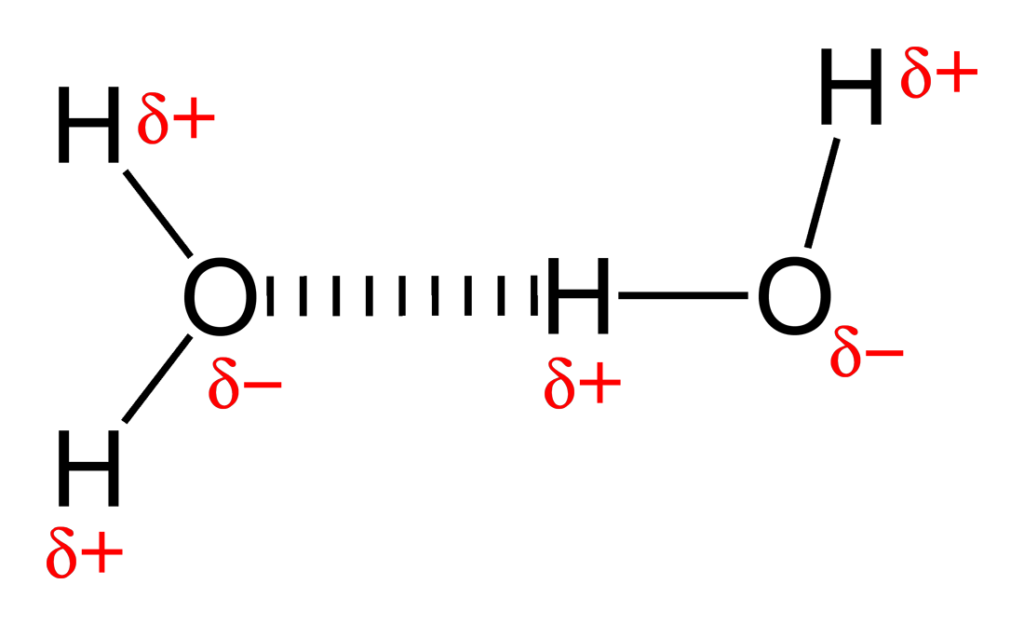
how to signify H bonds in a diagram
|||||||||||||||||||
rank intermolecular forces from weakest to strongest
LDFs < dipole-dipole < H bonds
explain H bonding
as a result of dipole-dipole interactions between H and atoms such as F, O or N
the H end of the molecule now has a partial +ve polarity
electron deficient H then attracts the lone pair of a nearby EN atom (that is of another molecule)
Difference between instantaneous dipole and dipole induced dipole
Instant dipole = is the movement of electrons within/between atoms of the same molecule
Dipole induced dipole = as a result of instant dipole in a nearby molecule, a dipole is induced in this molecule
between what type of molecules do dipole induced dipoles occur
between polar and non-polar molecules
TRUE OR FALSE: Instantaneous dipoles occur in both polar and non-polar molecules
True
State the Van der Waal forces
LDFs (all molecs)
dipole-dipole (polar molecs)
dipole induced (polar + non polar molecs tgthr)