Topic 3: Macromolecules
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
What are macromolecules
Large complex molecules
What are the four classes in the cell of macromolecules?
Carbohydrates *Polymer
Lipids
Nucleic acids *Polymer
Proteins *Polymer
What are polymers?
Molecules consisting of many similar or identical building blocks linked by covalent bonds
What are monomers?
Small molecule used as a building block for polymers
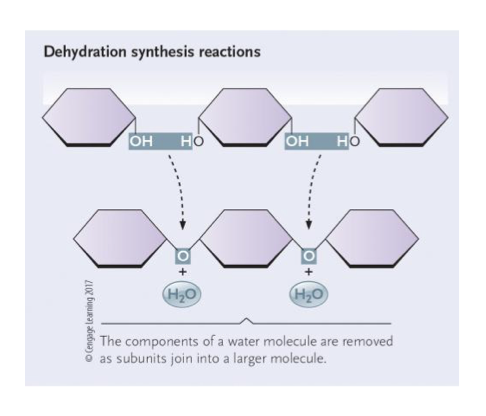
What happens during the synthesis of polymers (dehydration reaction)?
Covalent bond formed between monomers
Water molecule is lost
Requires energy
Requires enzymes
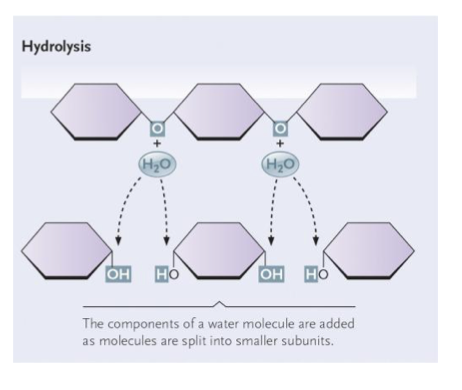
What happens during the degradation of polymers ((hydrolysis reaction)?
Breaks covalent bonds between two monomers
adds H2O
releases energy
requires enzymes
What are the functions of sugar and sugar polymers?
Source of energy
Source of carbon to make other molecules
Structural components of the cell
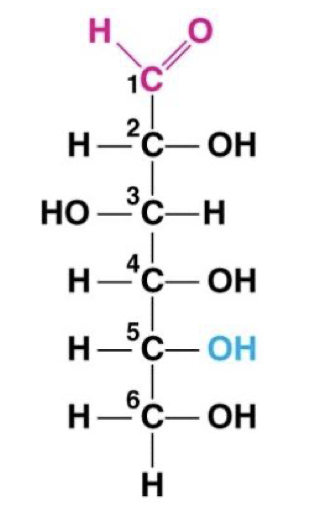
Monomer: monosaccharide
“Simple sugar”
One carbonyl and many hydroxyls (one per carbon)
Forms rings in solution
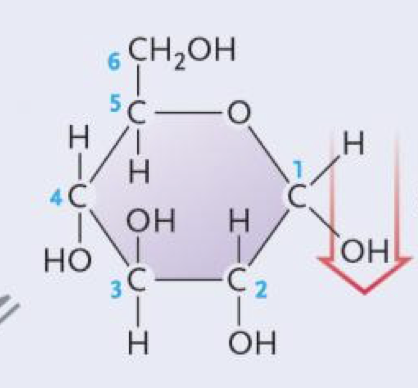
Alpha glucose
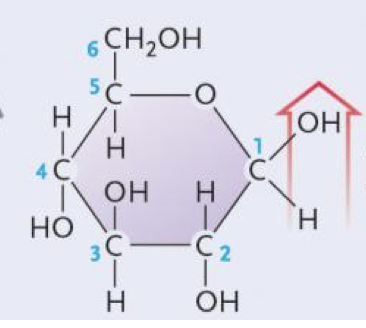
Beta glucose
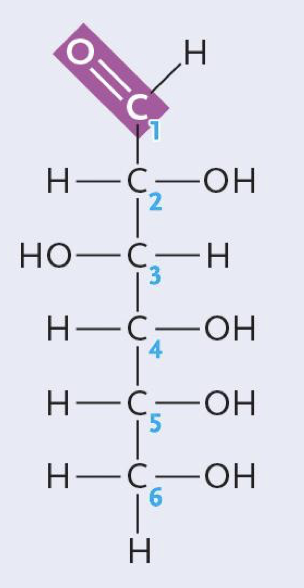
Glucose (aldehyde)
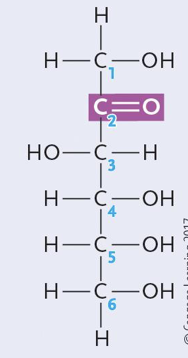
Fructose (a ketone)
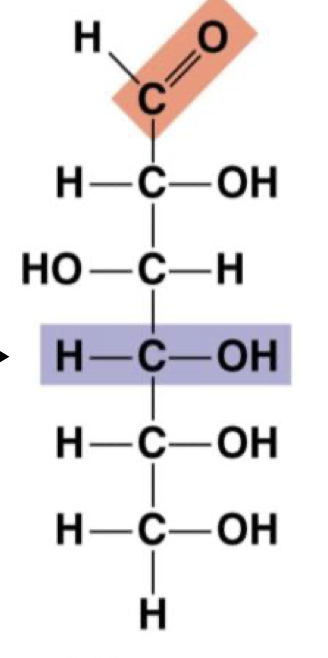
Glucose (enantiomers)
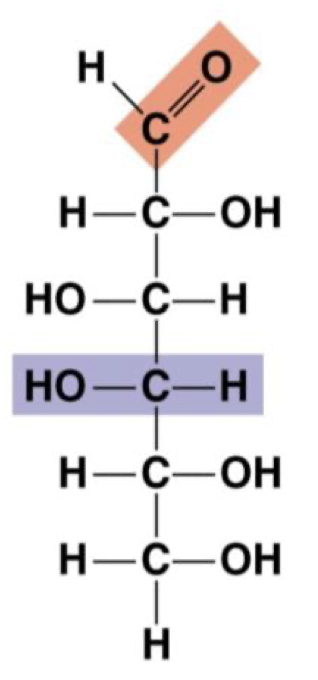
Galactose
Are glucose and fructose enantiomers?
No they are structural isomers
What are Monosaccharides joined by to form polymers?
Joined by bond called glycosidic linage, it Is a covalent bond and is formed by dehydration reaction
Polysaccharides
More than 2 monosaccharides joined by glycosidic linkages
-Polymer
-2 functions: energy storage and structure
What is starch (storage polysaccharide)?
-Only found in plants
-Polymer of glucose monomers joined by α-1,4-glycosidic bonds
-Helical structure
What is glycogen (storage polysaccharide)?
o Found in animal liver and muscle cells, and bacteria
o Glucose polymer with α-1,4-glycosidic bonds
o Helical structure
o Branched
What is cellulose (structural polysaccharide)?
Found in plant cell walls
Polymer of glucose with β-1,4-glycosidic linkages
Unbranched, linear structure (not helical)
Forms strong bundles
What are lipids in cells
Fats
Phospholipids
Sterols
Characteristics of Lipids
At least partially hydrophobic
Lots of non-polar bonds
Low water solubility
Not polymer (but still macromolecule)
What are the functions of fat?
Energy source, insulation, protection
What are the two components of fat?
1) Glycerol
-3 carbons
2) Fatty Acids
-3 hydroxyl
What is fat made out of?
1 glycerol + 3 fatty acids = triacylglycerol (attached by ester linkages)
How is it formed?
Formed by dehydration reaction
What are the function of phospholipids?
Main component of cell membrane
What are the characteristics of phospholipids?
Are amphipathic (part hydrophobic, part hydrophilic)
Similar to fat, except 3rd C of glycerol attached to phosphate group
Spontaneously assemble into bilayers
-Acts as boundary between call and environment
What are the functions of sterols?
Cell membrane, signalling molecules
What are the properties of sterols?
-Non-polar
-Carbon skeleton of 4 fused rings
Functions and characteristics of cholesterol
-Component of anime cell membrane
-Precursor to all other sterols
-Synthesized in liver; consumed in animal fats
-High levels may clog arteries
What is variation in fatty acids due to?
Length of H-C chain
#, location, and type of double bonds
Structure of Saturated Fatty acids
No Double bonds
Straight molecules
→Can pack close together
→Solid at room temperature
Ex: Saturated fats, red meat, butter
Structure of Unsaturated fatty acids
One or more double bonds
Bend at double bond
→Can’t pack closely together
→Liquid at room temperature
Ex: Unsaturated fat, oils, fish, plants
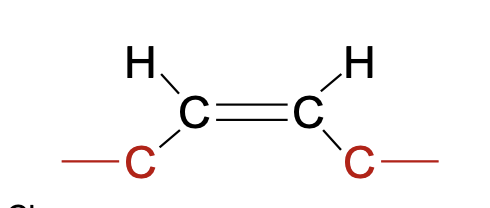
Cis fatty acids
Hydrocarbon chain at the same side
natural
bent molecule
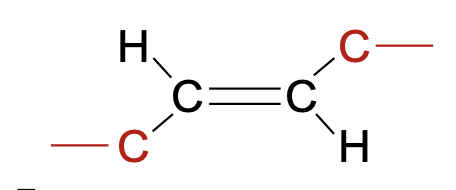
Trans fatty acid
Hydrocarbon chain opposite sides
Unnatural
Straight molecule
Hydrogenated oils
Hydrogen synthetically added to unsaturated dats to remove double bonds
Done because it becomes less perishable, ore solid at room temp, don’t separate
Examples: Margarine, peanut butter
What are the two types of nucleic acids?
DNA and RNA
What are the functions of DNA?
Genetic material of the cell
Contains all instructions for cell structure and function
Direct its own replication
Directs RNA synthesis
What are the functions of RNA?
Carries all information in our cells
Essential for protein synthesis
What is the composition of nucleic acid
Chain of nucleotides
Attached by phosphodiester linkage
→3’ -OH of one nucleotide attached to 5’ -P of the next nucleotide
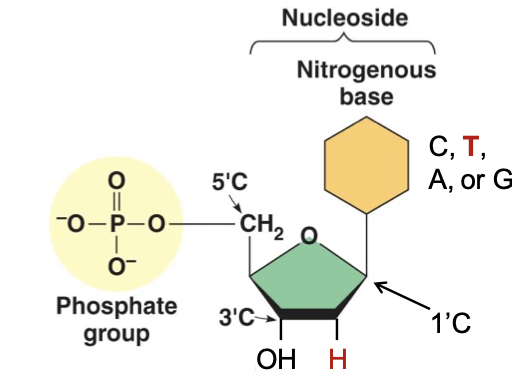
Deoxyribonucleotide
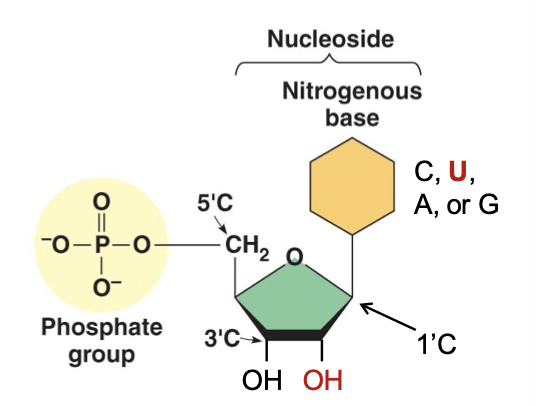
Ribonucleotide
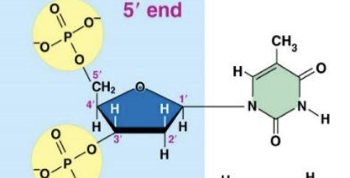
Thymine (T)
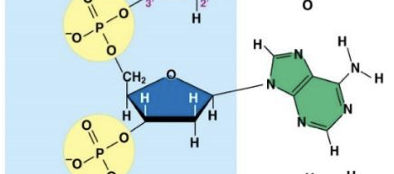
Adenine (A)
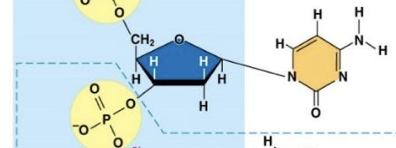
Cytosine (C)
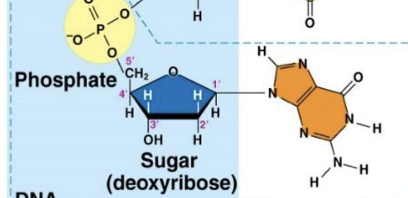
Guanine (G)
DNA structure
2 strands twist around each other to form double helix
fixed width of 2nm
phosphodiester backbone on outside of helix
nitrogenous bases are inside helix
fixed width of double helix means a purine (2 ring nitrogenous base) must pay with a pyrimidine (one ring)
Composition of strands in DNA?
Strands are specifically connected by H bonds between bases
→called base pairs
→2 contacts between A and T
→2 contacts between C and G (stronger
Two strands are antiparallel
RNA strand composition?
Exists as a single strand
Each has a unique shape due to internal base pairing
Still follow are pairing rules
→ A and U (2 H bonds)
→ G and C (3 H bonds)
How are protein in cells?
Hemoglobin, collagen, insulin etc
Functions of protein?
Enzymes
Transport proteins
Hormones
Receptors
Motor proteins
Structural proteins
Structure of amino acid (monomer)
-Central (alpha) carbon
-Amino group
-Carboxyl group
-R group = 20 different structures
-Ionized in the cell
Four classes of amino acids?
Non polar
Polar, uncharged
Polar, positively charged
Polar negatively-charged
Structure and formation of the Polymer: Polypeptide
Chain of amino acid monomers
Covalent bond called peptide bond
▪ Carboxyl group of one AA covalently joined to amino group of the
next AA
▪ Formed by a dehydration reaction
How can a polypeptide be a protein?
A polypeptide is a linear polymer of amino acid monomers connected by peptide bonds
A protein is a polypeptide that has been folded into a unique 3D shape
What is a protein?
One or more polypeptides folded together into a specific shape
Has four levels of protein structures that all occur simultaneously
Primary structure of protein
Unique order of amino acids in a polypeptide
• Bond type: peptide covalent
• Determined by DNA sequence
Secondary structure of protein
Repetitive coiling or folding of protein
• Bond type: H-bond in the polypeptide backbone
o Between amino group of one AA and carboxyl group of another AA
alpha helix structure
secondary structure and created by hydrogen bond in the backbone of polypeptide
-repetitive coiling
-H bond between every 4th amino acid in the helix
beta sheet
second secondary structure
repetitive folding
2 regains of peptide chain lie side by side and are connected by hydrogen bonds
Tertiary structure
• Overall shape of single polypeptide
• Due to interactions between
side chains
Hydrogen bond side chain location in tertiary structure
H bonds between polar side chains
Ionic bond side chain location in tertiary structure
Ionic bonds between oppositely charged side chains
Hydrophobic interaction side chain in tertiary structure
Non polar side chains aggregate inside protein and exclude water
Polar side chains exposed on protein surface and nteract with water
Disulfide bond side chain location in tertiary structure
__________________ between
the side chains of cysteine (AA) in _____________________
Quaternary structure
Multiple polypeptides folded together
o Each has its own 1°, 2°, and 3° structure
• Stabilized by same 4 types of side chain interactions as 3° structure (H-bonds, Ionic bonds, Hydrophobic interactions, Disulfide bonds)
• Protein may contain
o Multiple copies of same polypeptide; OR
o Several different polypeptides
How can protein have so many functions?
Protein shape is directly related to function
o All polypeptides have the same backbone
o Each has a different order of amino acids → controls shape → controls function
What might possibly when changes to the primary structure occur?
Changes can alter or eliminate protein function
o Different amino acid in primary sequence =
→ different side chain
→ forms different bonds (in tertiary structure)
→ changes overall shape
→ different shape = different/no function
What if a protein unfolds?
Denaturation: When protein unfolds and loses its normal shale
Causes a loss of function
Primary sequence conservative changes to protein
New AA has same properties as old AA
→ Similar bonds can form
→ Change has minimal effect on function
Primary sequence non-conservative changes to protein
New AA has different than old AA properties
→ Different bonds can form
→ Protein function is affected
What breaks interactions between side chains and causes a protein to denature?
High temperature
Change in pH
Organic solvents
Chemicals that disrupt bonds