Fluid Mechanics and Heat, Temperature, and Energy
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
Fluid Statics (Hydrostatics)
• Study of fluids at rest (not moving).
• Focuses on pressure, density, and buoyancy.
Fluid Dynamics
• S t u d y o f f l u i d s i n
motion.
• Focuses on flow rate,
Bernoulli’s principle,
and continuity equation.
Mass Density
A property of matter defined as the ratio of its mass (m) to its volume (V). It is denoted by the Greek letter rho (ρ) In equation form.
Pressure
A measure of the force exerted
per unit area.
increases
When the force increases, and the area stays the
same, pressure _________.
Decreases
When the area increases, and the force stays the
same, pressure _________.
Bouyancy
Upward force a fluid exerts on an object
Archimedes’ Principle
When an object is fully or partially immersed in a fluid, it experiences an upward buoyant force equal to the weight of the fluid
displaced by the object.
Sinks

Floats

Suspends/Partially Submerged

Daniel Bernoulli
Was a Swiss mathematician and physicist who discovered the relationship between the speed and pressure of fluids, known as Bernoulli’s Principle. His work helped explain how airplanes fly, blood flows, and how pressure changes in moving fluids.
Archimedes

Temperature
Defined as a measure of the average
kinetic of molecules making
up an object.
Heat
Is energy in transit from
one body to another due to a
difference in temperature.
Ralph H. Fowler
A British physicist and astronomer, formulated the Zeroth Law of
Thermodynamics in 1931.
Zeroth Law of Thermodynamics
It states that two bodies are in thermal equilibrium if they have the same temperature
Thermal equilibrium
Is the condition in which two or
more objects in contact no longer exchange heat
because they have reached the same temperature.
Temperature scale
A system for measuring how
hot or cold something is. It’s based on reference points
and divisions between them. The most common scales
are:
First Law of Thermodynamics
Energy cannot be created or
destroyed. It can only change
forms.
Second Law of Thermodynamics
Heat always flows from hot to cold. Things naturally become more disordered.
“In an isolated system, entropy increases,
and the energy becomes more disordered”
Third Law Law of Thermodynamics
At absolute zero (0 K), a perfect
crystal has no disorder; everything
stops moving.
Physical Change
When a substance
changes its former form or appearance, but no new substance is made.
• The change is usually
reversible.
• T h e c h e m i c a l
composition stays the
same.
Chemical Change
When a substance changes into a new substance with different
properties.
• The change is usually
irreversible.
• The chemical bonds
b e t w e e n a t o m s a r e
broken or formed.
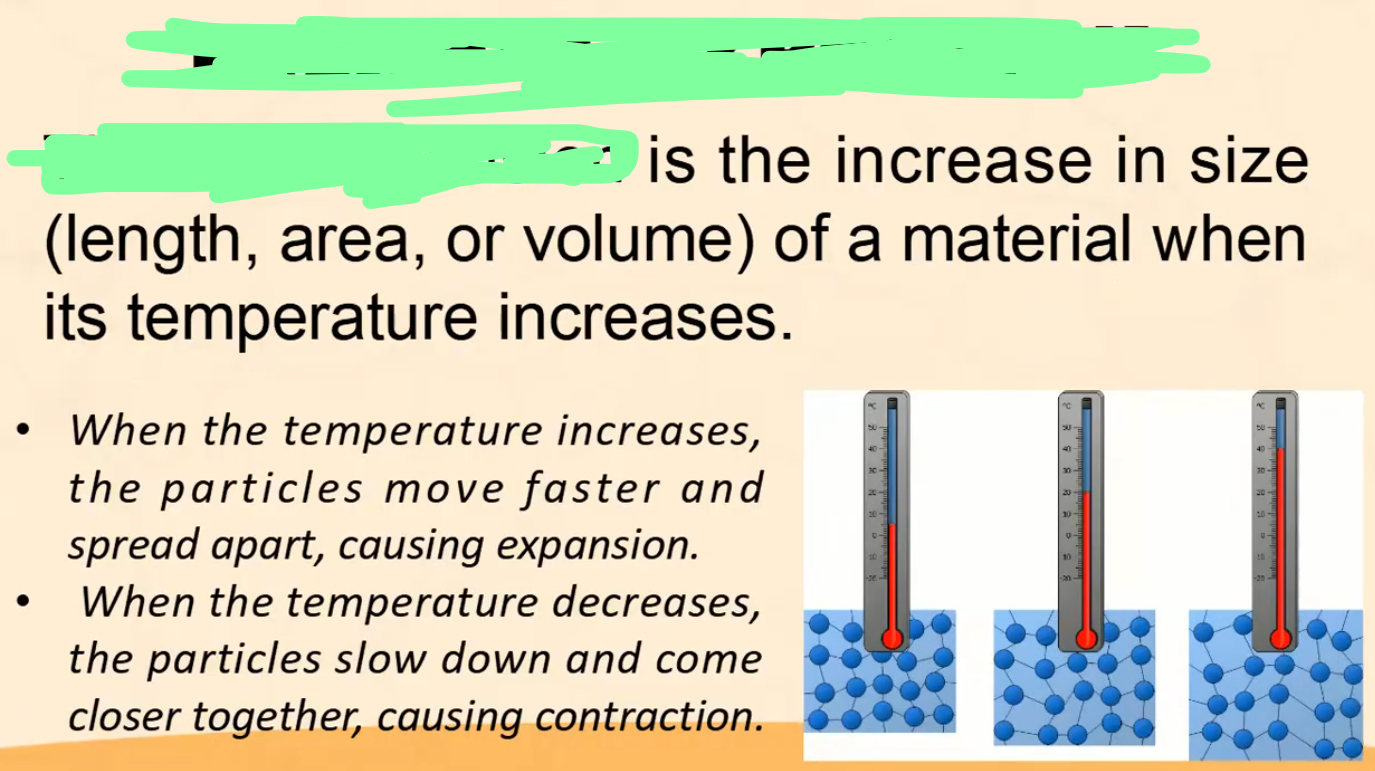
Thermal Expansion
Latent heat

Isothermal Process
