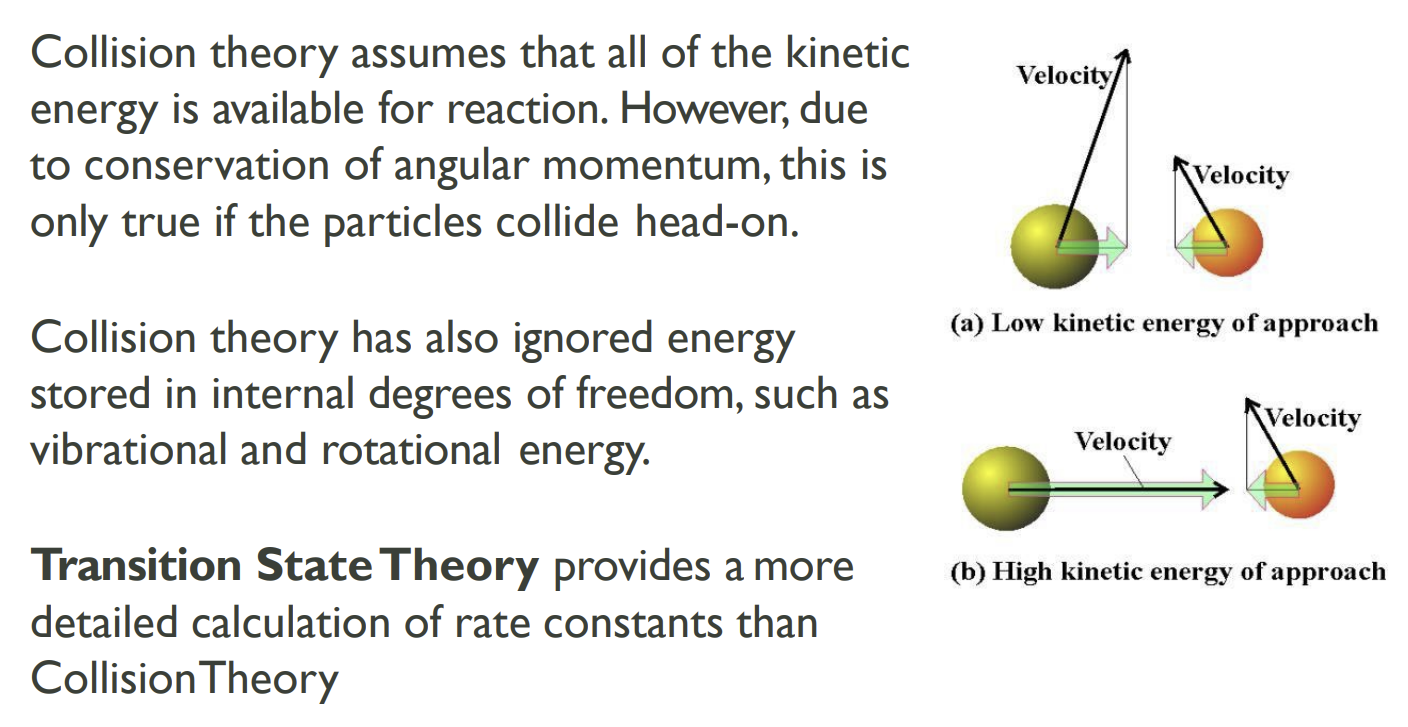
Collision theory
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
What things are required for a reaction according to collision theory
A collision, meeting the energy requirement, meeting the steric requirement.
Collision Cross-Section
Imagine all but one molecule is held still and this molecule moves in a straight line.
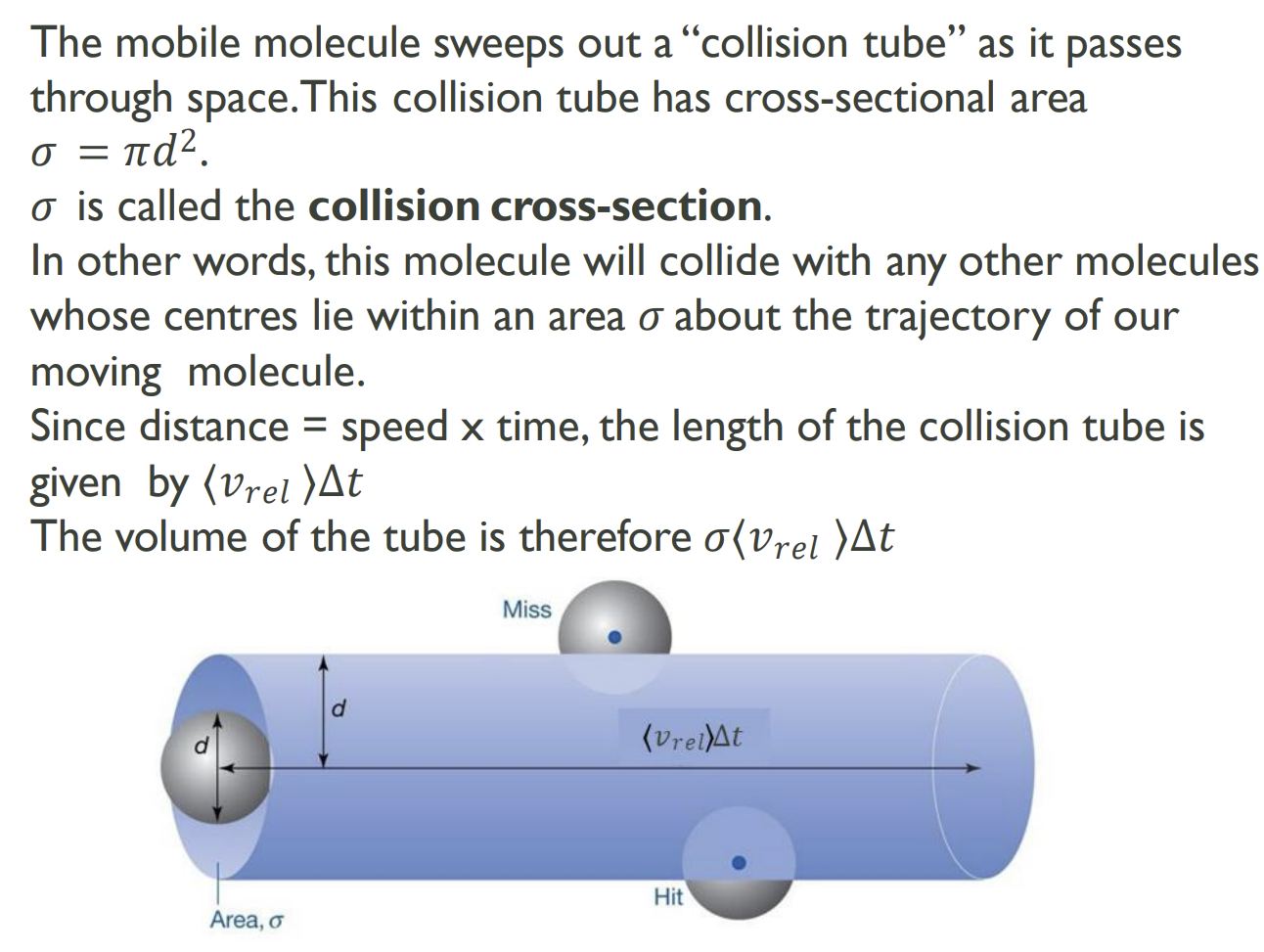
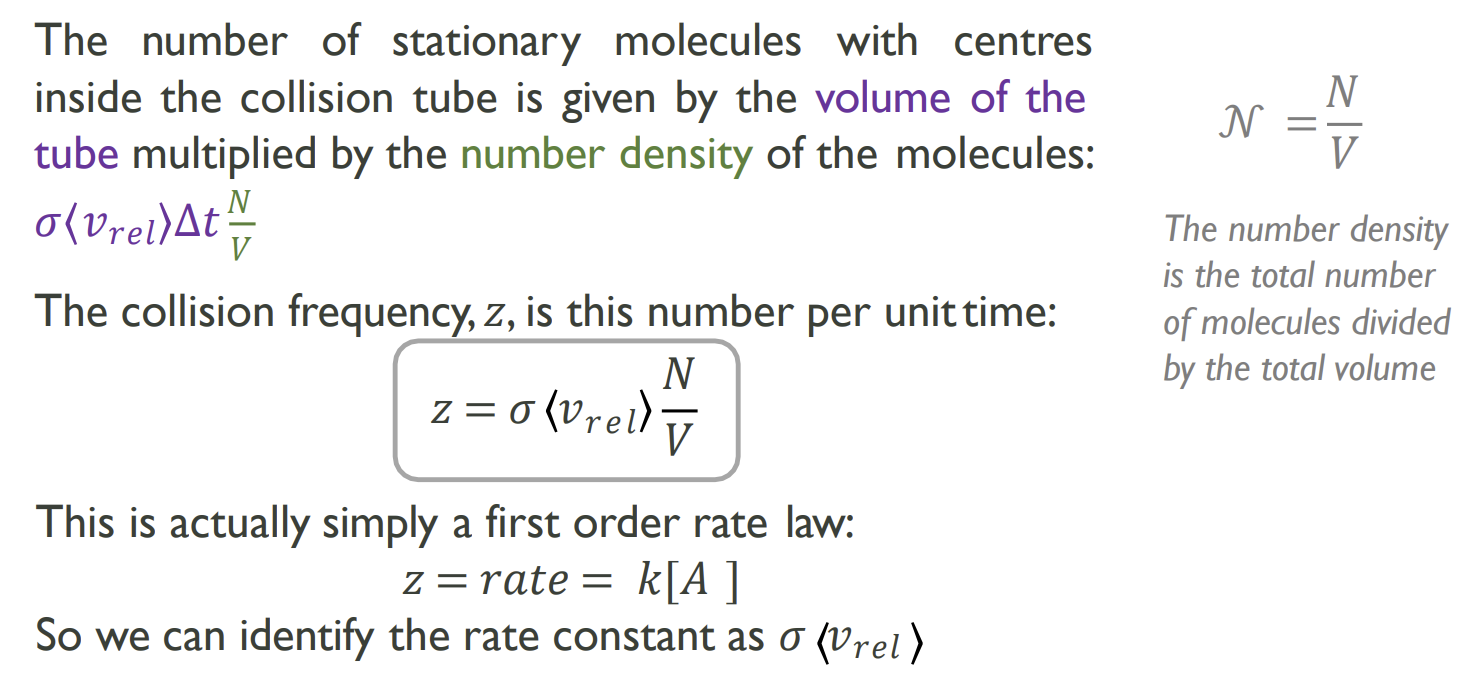
How is collision frequency, z, calculated
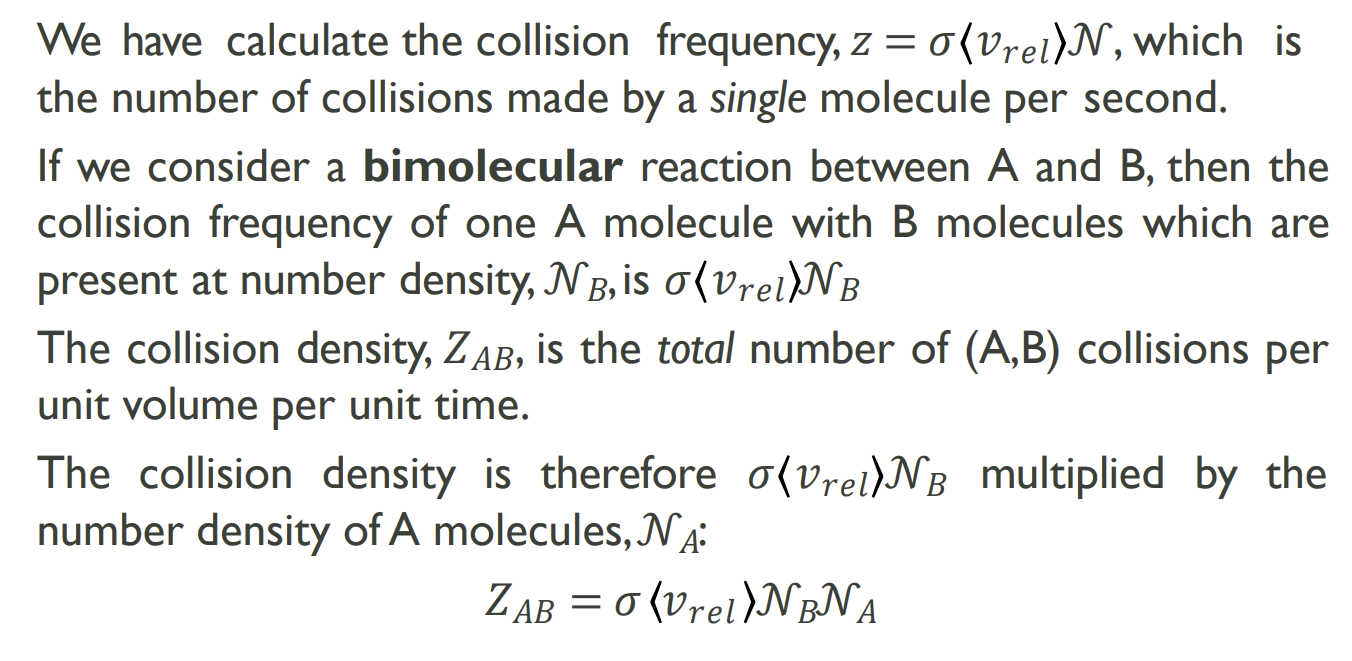
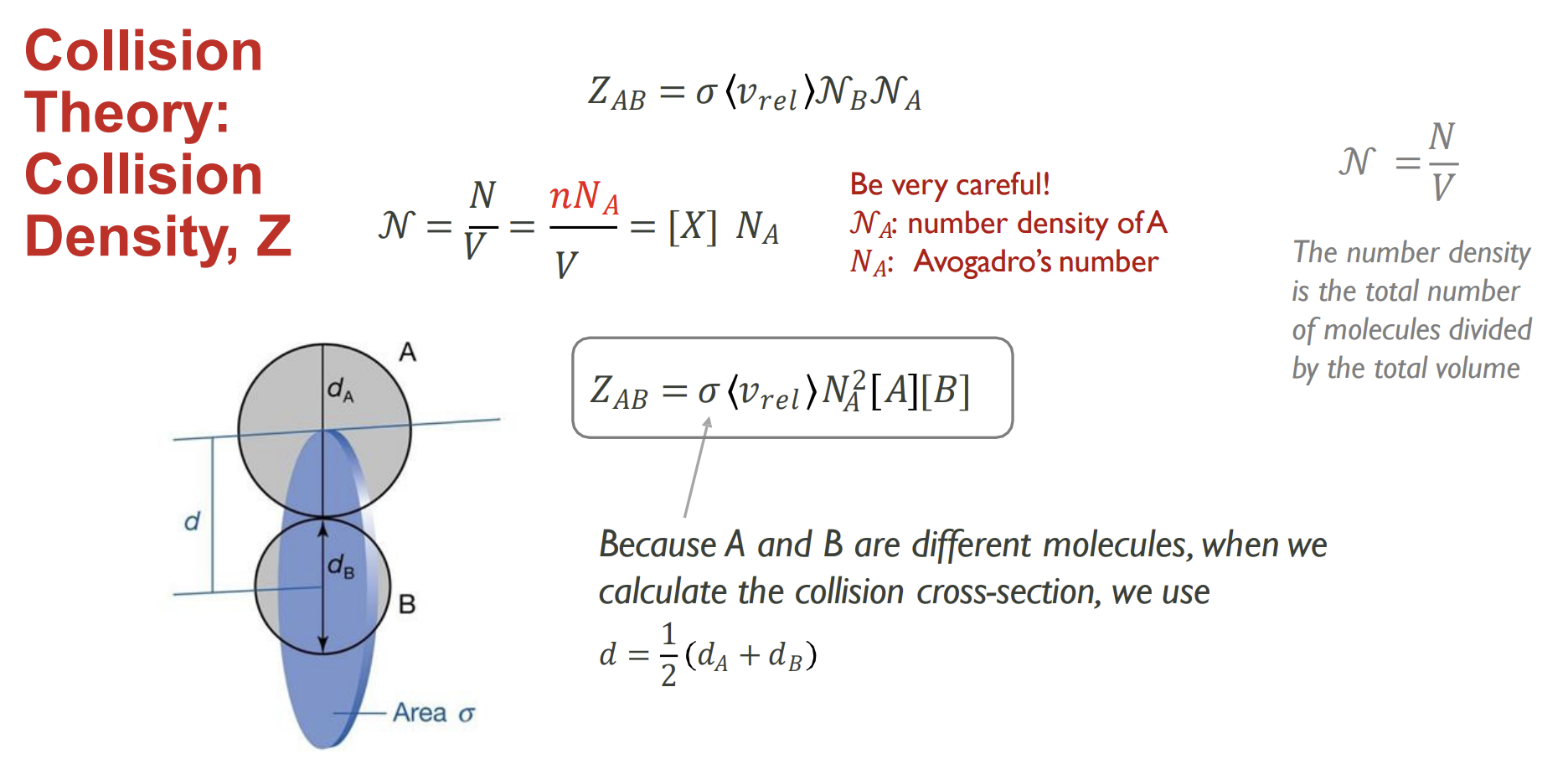
How do you calculate collision density, Z and how is it different
Taking the calculation from the previous slide and multiplying by the number density of A allows you to take all molecules of A into account at once. Number density includes the collisions of every molecule of A
How do you relate number density to concentration
little n is number of moles as usual
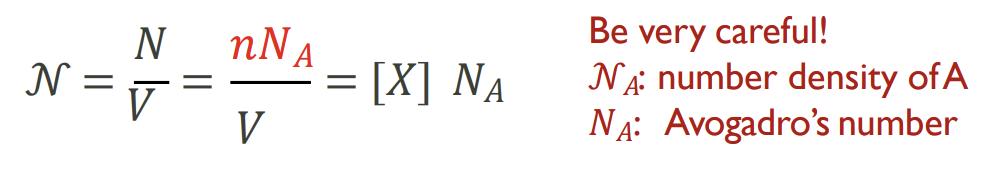
How can we calculate collision density using concentrations of different molecules
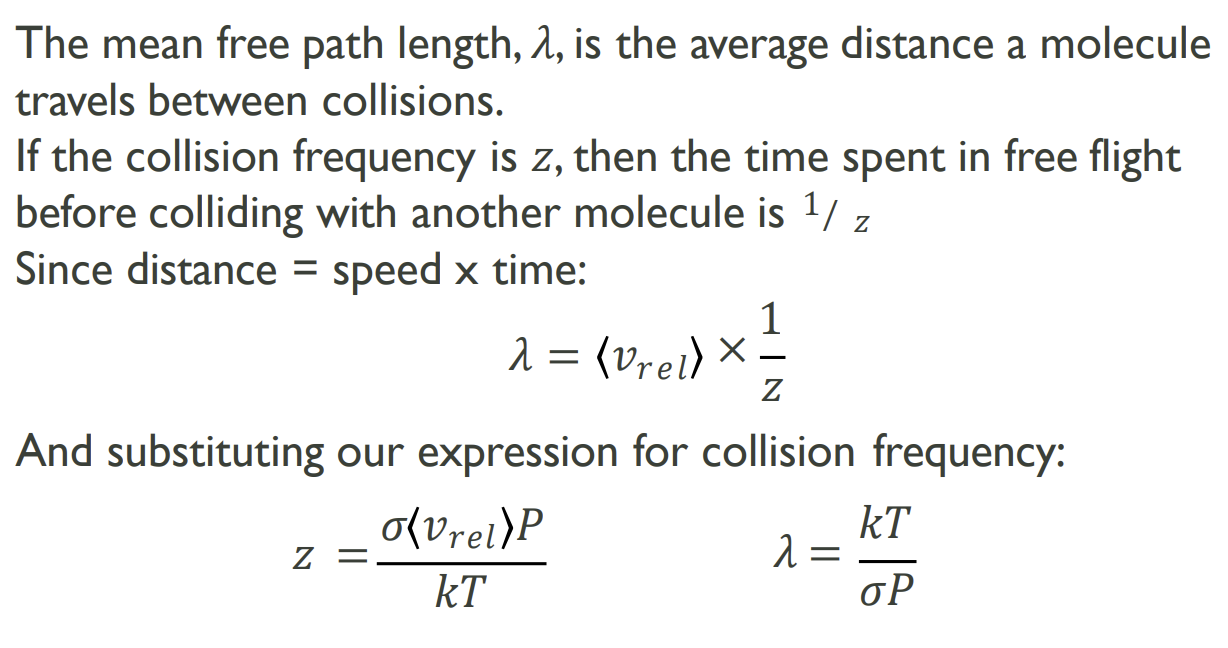
What is the Mean Free Path Length and how is it calculated
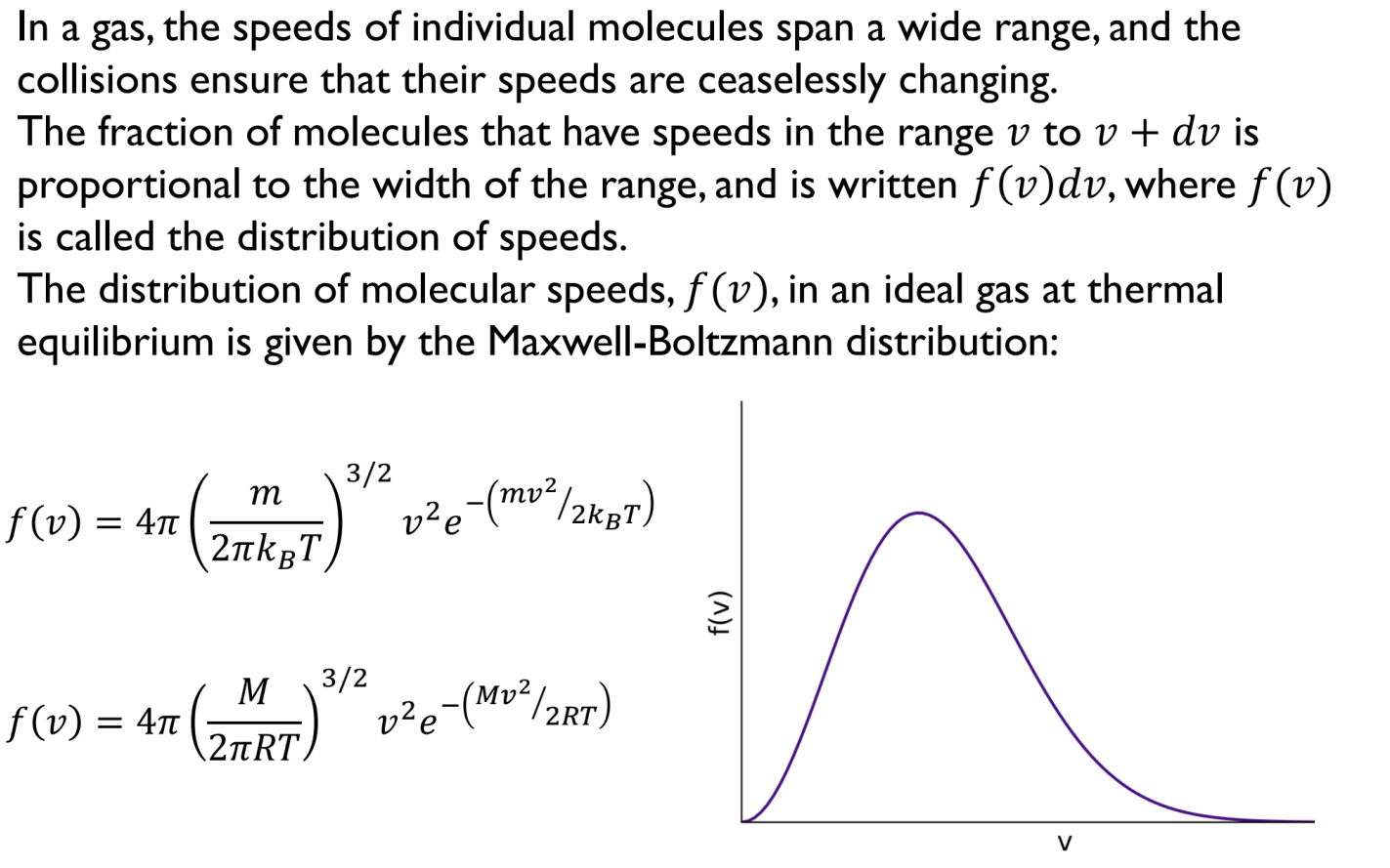
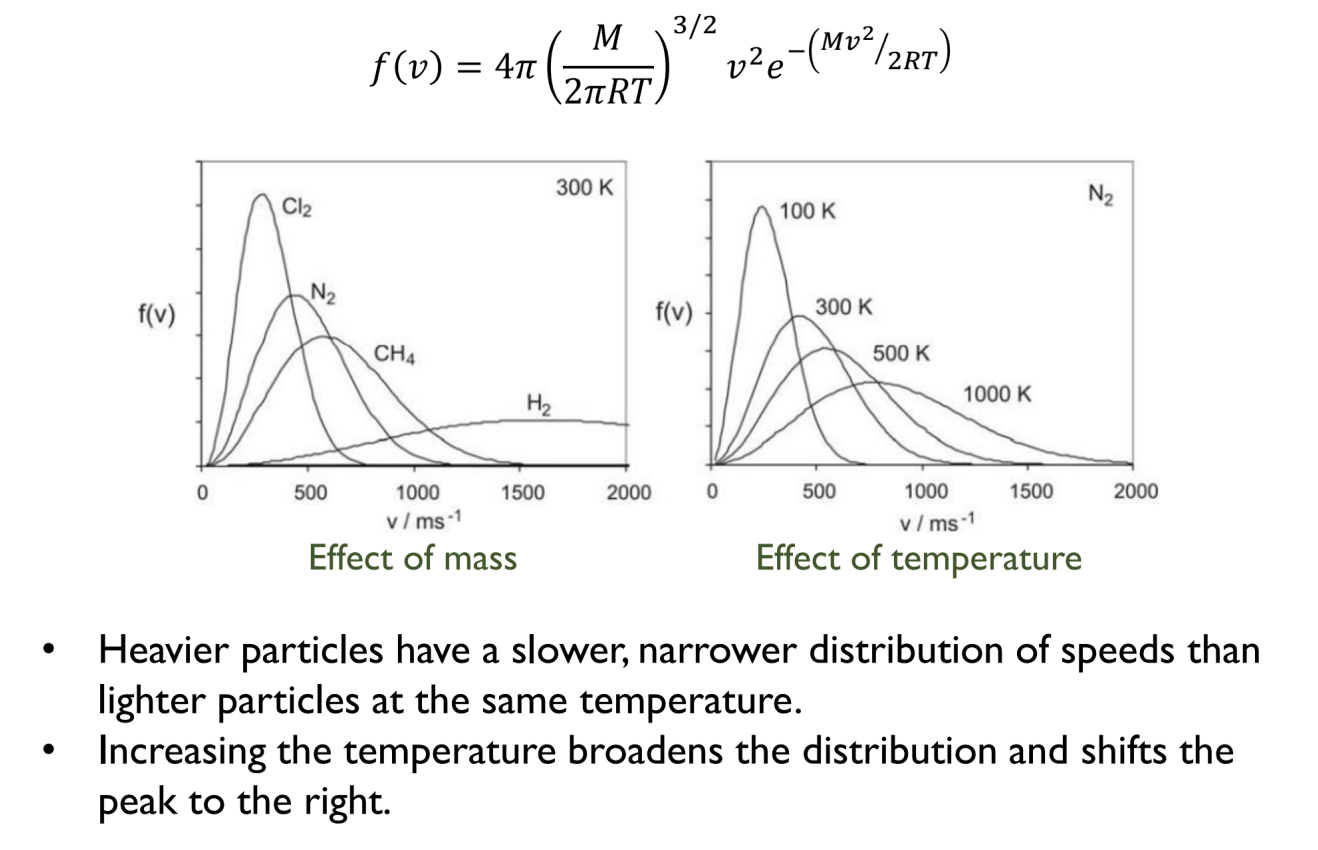
Maxwell-Boltzmann Distribution of Speeds
Effect of mass and temperature on the distribution
Equations to remember: Mean speed
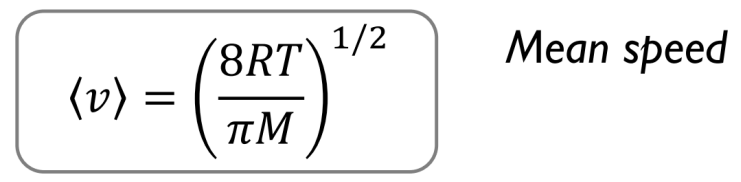
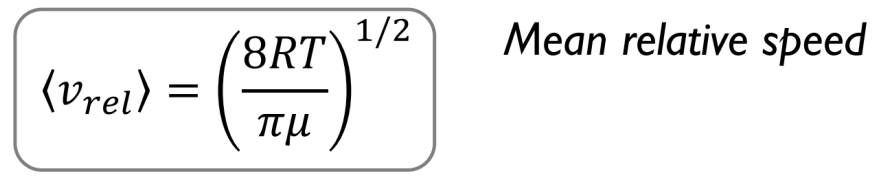
Mean relative speed equation
Most probable speed

Root mean square speed
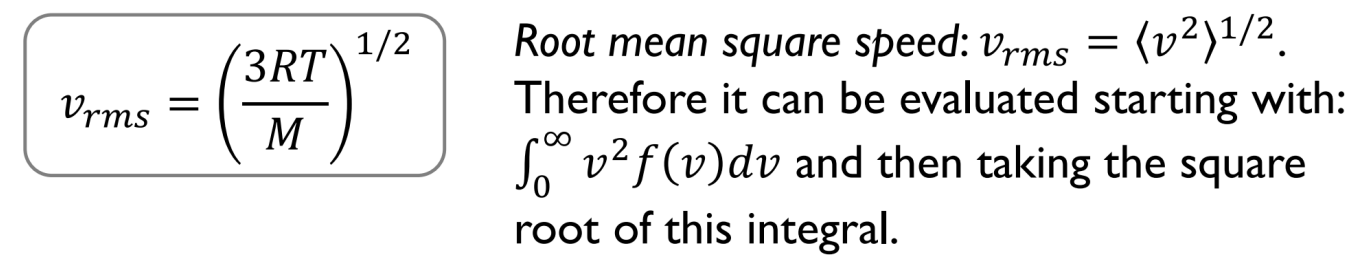
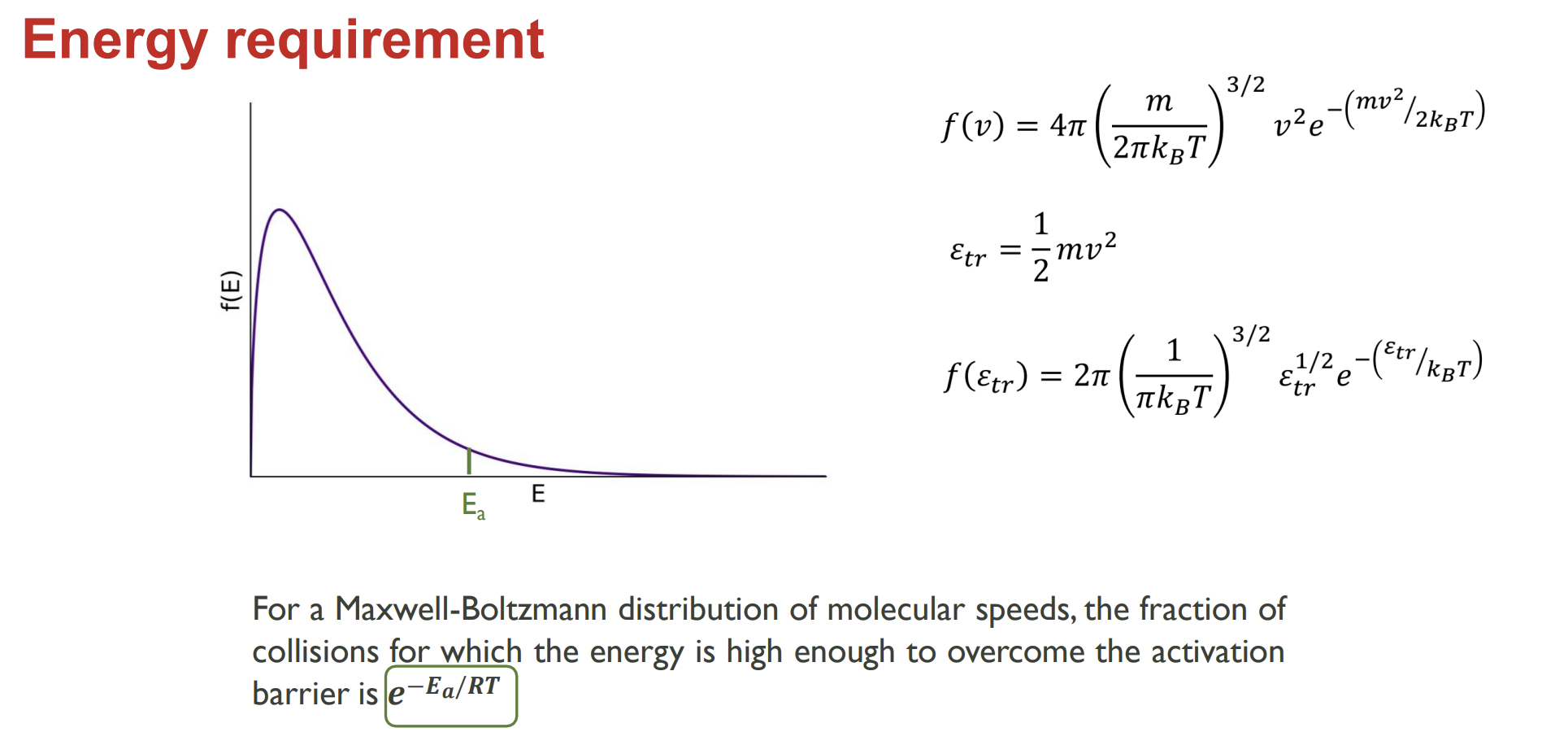
Energy distribution
Steric requirement component
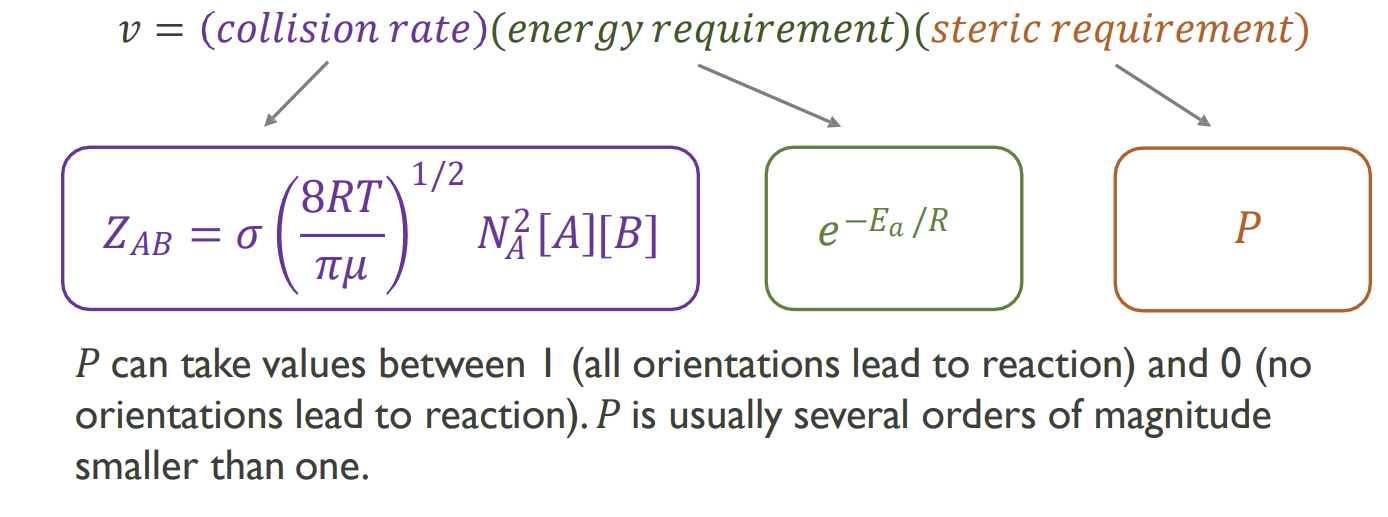
How can the cross section be modified to account for steric requirements

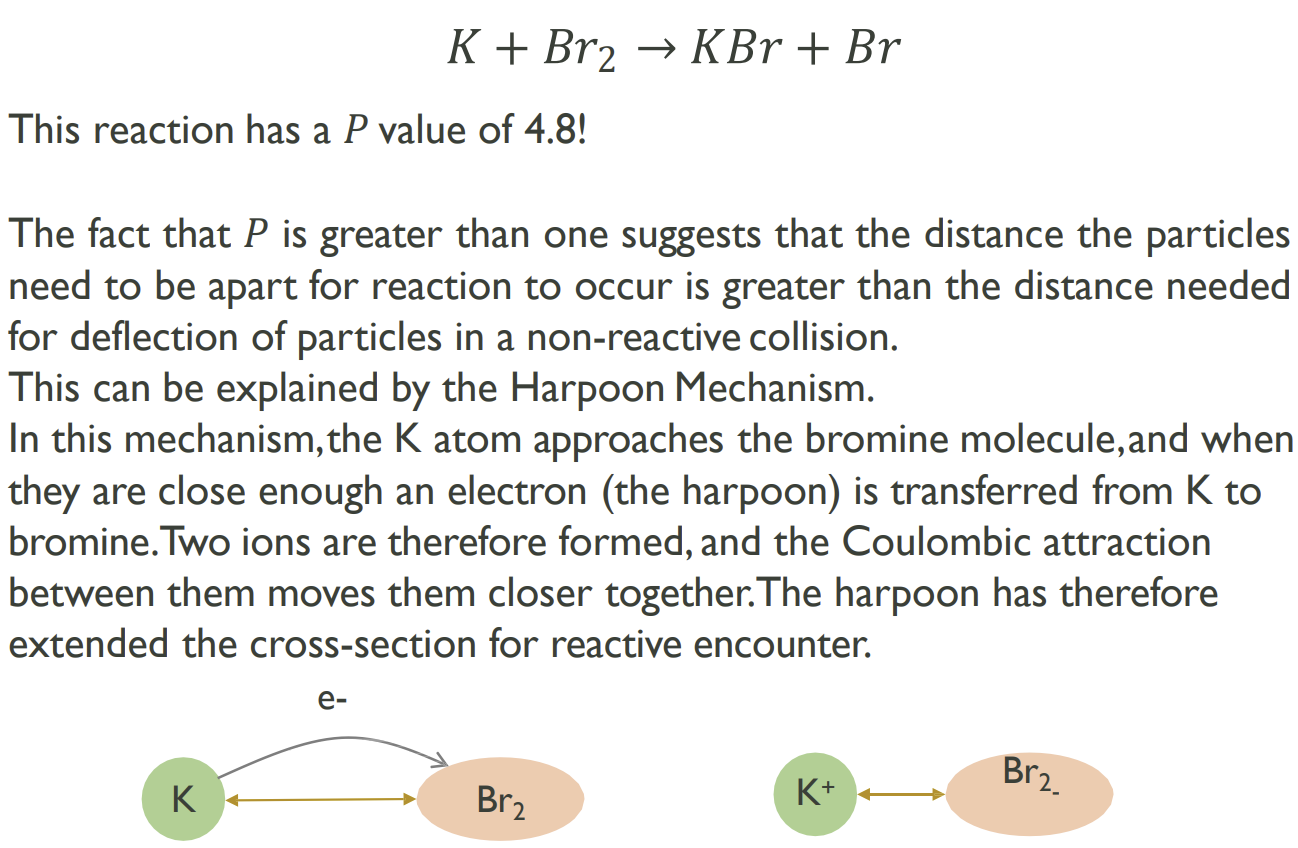
The Harpoon Mechanism - an exception with sterics
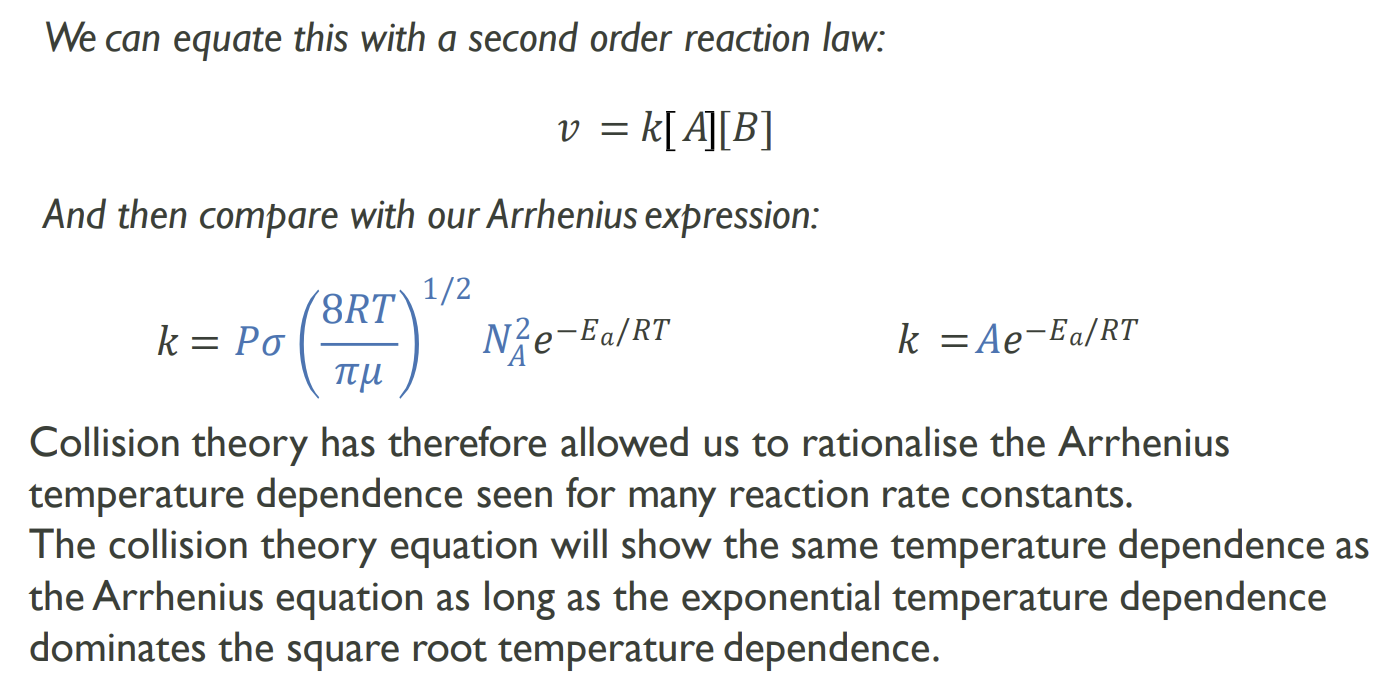
How can the overall rate equation be expressed
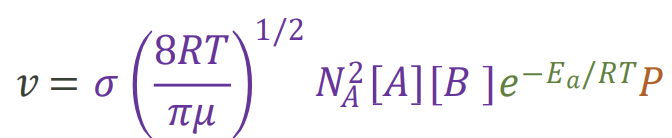
How can this equation give us the Arrhenius equation
Limitations of collision theory