Chapter 10: Chemical Bonding 1- The Lewis Model
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
51 Terms
Lewis model
A simple model of chemical bonding using diagrams that represent bonds between atoms as lines or pairs of dots. According to this model, atoms bond together to obtain stable octets (eight valence electrons).
Lewis electron-dot structures (Lewis structures)
A drawing that represents chemical bonds between atoms in molecules as shared or transferred electrons; the valence electrons of atoms are represented as dots in a Lewis structure.
ionic bond
A chemical bond formed between two oppositely charged ions, generally a metallic cation and a nonmetallic anion, that are attracted to each other by electrostatic forces.
covalent bond
A chemical bond in which two atoms share electrons that interact with the nuclei of both atoms, lowering the potential energy of each through electrostatic interactions.
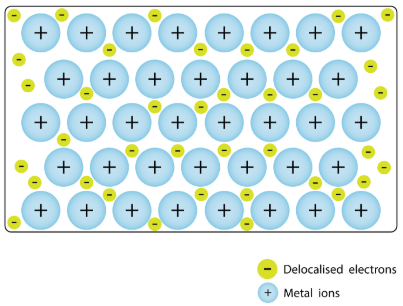
metallic bonding
The type of bonding that occurs in metal crystals, in which metal atoms donate their electrons to an electron sea, delocalized over the entire crystal lattice.
Lewis symbol
The symbol of an element surrounded with dots representing the element’s valence electrons.
octet
The eight dots around atoms in a Lewis structure that signify a filled outer electron shell for s and p block elements.
duet
A Lewis symbol with two dots, signifying a filled outer electron shell for the elements H and He.
chemical bond
The sharing or transfer of electrons to attain stable electron configurations for the bonding atoms.
octet rule
The tendency for most bonded atoms to possess or share eight electrons in their outer shell to obtain stable electron configu-rations and lower their potential energy.
lattice energy
The energy associated with forming a crystalline lattice from gaseous ions.
Born-Haber cycle
A hypothetical series of steps based on Hess’s law that represents the formation of an ionic compound from its constituent elements.
bonding pair
A pair of electrons shared between two atoms.
lone pair
A pair of electrons associated with only one atom.
nonbonding electrons
Electrons in a Lewis structure that are not in a chemical bond; also called lone pair electrons.
double bond
The bond that forms when two electrons are shared between two atoms.
triple bond
The bond that forms when three electron pairs are shared between two atoms.
polar covalent bond
A covalent bond between two atoms with significantly different electronegativities, resulting in an uneven distribution of electron density.
electronegativity
An atom’s ability to attract electrons to itself in a covalent bond.
dipole moment (u)
A measure of the separation of positive and negative charge in a molecule.
percent ionic character
The ratio of a bond’s actual dipole moment to the dipole moment it would have if the electron were transferred completely from one atom to the other, multiplied by 100%.
resonance structures
Two or more valid Lewis structures that are shown with double-headed arrows between them to indicate that the actual structure of the molecule is intermediate between them.
resonance hybrid
The actual structure of a molecule that is intermediate between two or more resonance structures.
formal charge
The charge that an atom in a Lewis structure would have if all the bonding electrons were shared equally between the bonded atoms.
free radical
A molecule or ion with an odd number of electrons in its Lewis structure.
bond energy
The energy required to break 1 mol of the bond in the gas phase.
bond length
The average length of a bond between two particular atoms in a variety of compounds.
Why do atoms form chemical bonds?
atoms are trying to reach the most stable (lowest-energy) state that they can
According to the Lewis model, what do the dots in a Lewis structure represent?
valence electrons
What type of bond involves the transfer of electrons from one atom to another?
ionic
In the molecule H₂O, what is the reason it forms with two hydrogen atoms rather than three (H₃O)?
it allows both atoms to achieve a stable electron configuration by sharing electrons
Which of the following compounds is predicted to have the highest lattice energy?
a) NaCl
b) MgO
c) KBr
d) CaS
MgO
→ Higher charges (Mg²⁺ and O²⁻) and smaller ions = stronger electrostatic attraction = higher lattice energy.
Which type of bond involves the delocalization of electrons across many atoms?
Metallic
Which molecule is most likely to have a polar covalent bond?
a) O₂
b) HCl
c) N₂
d) Cl₂
HCl
→ Large difference in electronegativity between H and Cl makes it polar covalent.
In terms of electronegativity, which bond is the most polar?
a) H–F
b) C–H
c) N–O
d) O–Cl
H–F
→ H (2.1) and F (4.0) have the biggest electronegativity difference.
What is the bond angle in a molecule with tetrahedral electron geometry?
109.5°
T/F: The Lewis model predicts that ionic solids conduct electricity in the solid state.
False
→ Ionic solids only conduct electricity when melted or dissolved, not as solids.
T/F: A molecule with resonance structures actually oscillates between different forms.
False
→ Resonance is not back-and-forth flipping; it’s a blend of all structures.
T/F: A triple bond is generally shorter and stronger than a single bond.
True
→ Triple bonds are shorter and stronger than single bonds.
T/F: Formal charge should always be placed on the least electronegative atom.
False
→ Formal charges should ideally be placed on the most electronegative atoms.
T/F: Hydrogen can form more than one covalent bond to fulfill its octet.
False
→ Hydrogen can only form one bond (1s¹ – wants 2 electrons total, not 8).
Explain why water is H₂O and not H₃O using the Lewis model.
→ Oxygen has 6 valence electrons and forms 2 bonds to complete its octet. H₃O⁺ exists but only with a positive charge, and in H₂O, oxygen has 2 bonding pairs and 2 lone pairs.
Describe how the Lewis model explains why ionic compounds are brittle.
→ The Lewis model explains brittleness because when the lattice is disturbed, like charges align and repel each other, causing the crystal to fracture.
Define electronegativity and explain how it affects bond polarity.
→ Electronegativity is an atom’s ability to attract electrons in a bond. Larger differences in electronegativity between atoms cause polar bonds.
What’s the relationship between bond length and bond energy?
→ Shorter bonds = stronger = higher bond energy.
Long bonds (like single bonds) are weaker, while triple bonds are shorter and stronger.
List three exceptions to the octet rule and briefly explain each.
Incomplete octet: e.g., Boron in BH₃ (only 6 electrons).
Expanded octet: e.g., PCl₅, SF₆ – atoms in period 3 or beyond can hold more than 8.
Odd-electron species (radicals): e.g., NO, which has an unpaired electron.
Draw the Lewis structure for CO₂. Indicate any double bonds and lone pairs. (just sketch it urself and the answer will be on this as an image, just type a period as your answer to see it)
.

Draw all valid resonance structures for the nitrate ion (NO₃⁻). Use formal charges. (just sketch it urself and the answer will be on this as an image, just type a period as your answer to see it)
.
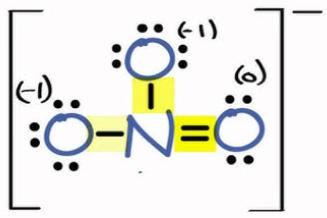
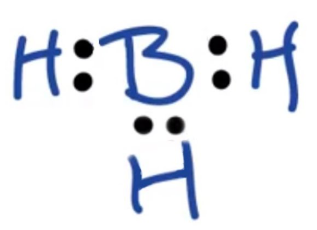
Draw a Lewis structure for BH₃. Is this molecule an exception to the octet rule? Explain. (just sketch it urself and the answer will be on this as an image, just type the explanation answer)
→ Yes, it’s an exception to the octet rule. Boron only has 6 electrons.
Draw a molecular-level sketch of a metallic bond and label the 'sea of electrons'. (just sketch it urself and the answer will be on this as an image, just type a period as your answer to see it)
.
Use average bond energies to estimate ΔH for this reaction:
CH₄ + Cl₂ → CH₃Cl + HCl\text{CH₄ + Cl₂ → CH₃Cl + HCl}CH₄ + Cl₂ → CH₃Cl + HCl
(Use standard bond energies for: C–H = 413 kJ/mol, Cl–Cl = 243 kJ/mol, C–Cl = 328 kJ/mol, H–Cl = 431 kJ/mol)
Bonds broken:
4 C–H bonds in CH₄: 4 × 413 = 1652 kJ
1 Cl–Cl: 243 kJ
Total energy in (breaking bonds): 1652 + 243 = 1895 kJ
Bonds formed:
3 C–H (still there in CH₃Cl): 3 × 413 = 1239 kJ
1 C–Cl: 328 kJ
1 H–Cl: 431 kJ
Total energy out (forming bonds): 1239 + 328 + 431 = 1998 kJ
ΔH ≈ bonds broken – bonds formed =
1895 – 1998 = –103 kJ (exothermic)