Cancer Bio Final
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
62 Terms
What percentage of cancers are caused by viruses?
15 - 25%
Peyton Rous
Known for Rous protocol for inducing sarcomas in chickens
Chicken with sarcoma in breast muscle
Remove sarcoma and grind up tissue, collect fine filtrate
Inject filtrate into young chicken
Observe sarcoma in infected chicken
Rous Sarcoma Virus (RSV)
Johannes Andreas Grib Fibiger
Studied how specific viruses caused stomach cancer in rats
Spiroptera carcinoma - Roundworm parasite of rats
Possible link between parasite and stomach cancer
Controversial
Others recreated the experiment to different results
Renato Dulbecco
Italian-American Virologist who won nobel prize in 1975 for his work on oncoviruses
First to grow cells in a petri dish
Genes from viruses incorporated into host, cause mutations in host making them cancer cells
Howard M. Temin
Convinced that he wants to find that what was in the filtrate was a virus
Starts with RSV (Rous Sarcoma Virus)
RSV causes cell transformations
Infected with RSV particle
Transformation of a cell
Multiplication
Anchorage - independent growth on soft agar
Normal cells can’t anchor, die, but cancerous cells don’t need and can grow on soft agar
What was revolutionary about Howard Teemin’s discovery
Temin hypothesized that retroviruses used a DNA intermediate (provirus) to integrate their RNA genomes into host cells
Temin and David Baltimore discovered reverse transcriptase, and enzyme which confirmed this hypothesis and allows integration by creating DNA from RNA
Nude Mice
Growth of human ear on back of a mouse
Temin made sarcomas on mice
People started working on other viruses and found support
Temin RSV Experiment
Focused on if viruses have to be present at all times to cause mutations
Conclusion: Yes
Higher temp causes virus to be deactivated,
RSV must be present at all times to maintain cells transformations
Properties of transformed cells
Altered morphology/shape
Loss of contact inhibition
Ability to grow without attachment (anchorage independence)
Reduced requirement for mitogenic growth factors
High saturation density
Inability to halt proliferation in response to deprivation of growth factors
Increased transport of glucose
Integration of retroviruses into chromosomal DNA
1) Entry into cell and shedding of envelope
2) Reverse transcriptase (DNA/RNA and then DNA/DNA)
3) Integration of DNA copy into host chromosome
4)Integrated DNA (=provirus) transcribed and translated into proteins —> Many new virus particles
Types of Retroviruses
Transforming vs non-transforming
Transforming: Carry viral oncogenes → Rapid tumor development
Non-transforming: Don’t carry oncogenes, longer period of inducing tumors by inserting themselves within or near oncogenes
ALV- Infection but no-transformation
RSV-Infection and transformation
Michael Bishop / Harold Varmus and RSV, src
Studying RSV
RSV contains a gene called v-src that is responsible for transforming normal cells into cancerous ones
These genes are actually mutated forms of normal genes (c-src, which is a proto-oncogene)
c-src (proto-oncogene) is kidnapped by the RSV to cause cell transformation
List four oncogenes and how they become oncogenes
src (Rous sarcoma virus)
non-receptor TK (Tyrosine kinase)
Sarcoma (chickens)
ros (UR2)
RTK, unknown ligand
Sarcoma (chickens)
myc (myelocytomatosis 29)
Transcription factor
Myeloid leukemia (Chickens)
akt (AKT8)
ser/thr kinase
lymphoma (mouse)
What ideas came from retrovirus discovery?
1) If retroviruses could
All transforming powers of RSV derived from presence of a single gene
Maybe other transforming retroviruses had acquired other cellular genes unrelated to src
c-myc and viruses
Encodes for a transcription factor (MYC) that regulates numerous other things related to cell growth
some retroviruses, such as avian leukosis virus (ALV),
ALV integrates its DNA randomly into a promotor / regulator region of c-myc, upregulating c-myc (insertional mutanogenesis)(expression and making it over expressed
Some viruses carry a mutated copy
Result = uncontrolled proliferation due to over production of MYC
src and its role in cell signaling
a non-receptor tyrosine kinase, plays critical role in cell signaling
Starts pathway that recruits AKT to the membrane, AKT fully phosphorylated
phosphorylates targets like
GSK-3β (promoting proliferation)
BAD (inhibiting apoptosis)
HIF-1α (promoting angiogenesis)
Viral inactivation of p53
HPV (Human papilloma virus) 16 and 18 high risk, innactivates p53
adeno E1B blocks transcriptional acctivation
Also inactivation of Rb protein fn (tumor supressor) regulating TFs like E2F
Few more oncogenes
Name of v, oncogene, type of oncoprotein, cancer in humans
Harvey sarcoma, (H-ras), small G protein, bladder carcinoma. OG in rodents
Kirsten sacroma, (K-ras), small protein, many types of cancer, og in rodents
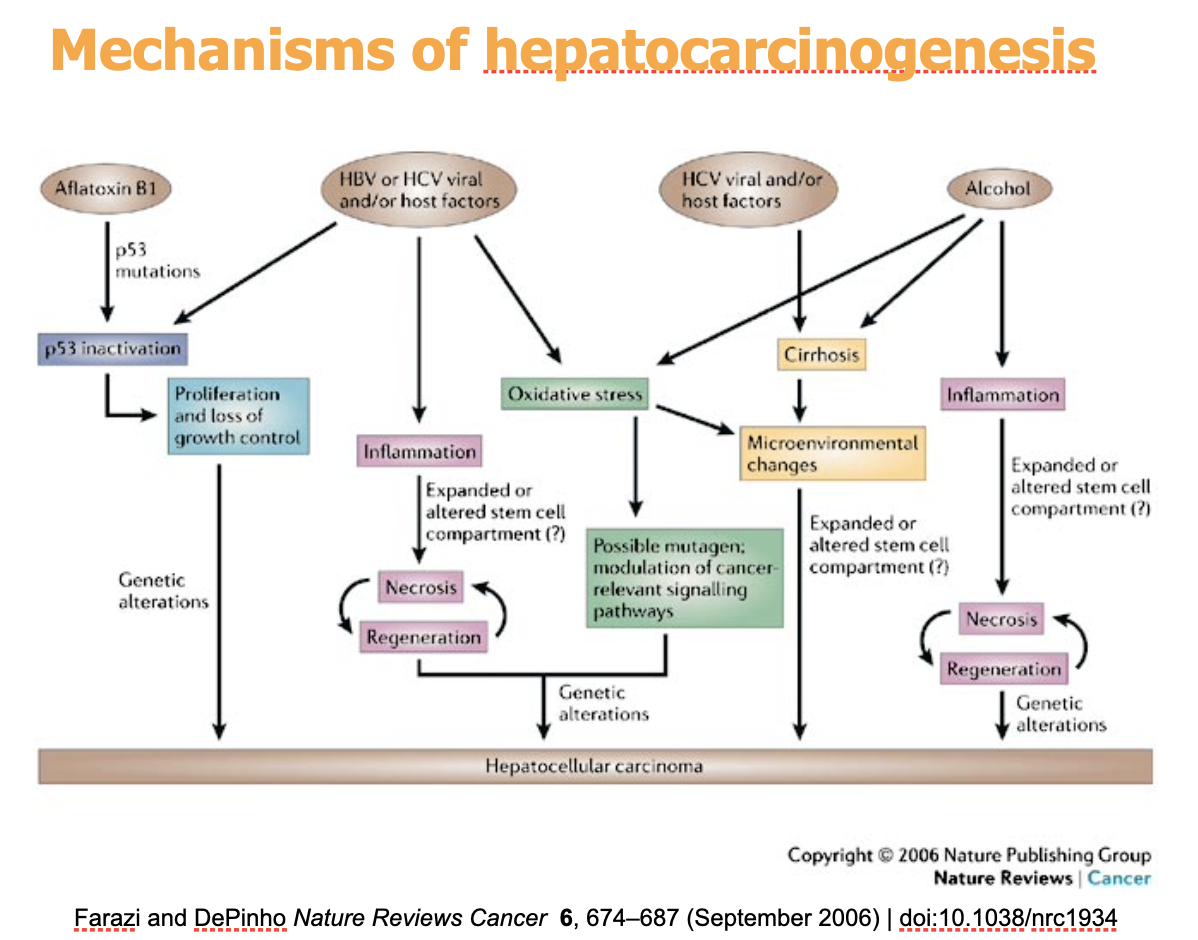
Mechanisms of hepatocarcinogenesis
1) Chronic infection or damage from HBV, HCV, Alcohol, Alfatoxin B1 (comes from fungi)
2) Liver cirrhosis and DNA damage
3) genomic instability and loss of p53 (Aflatoxin B1 big role in loss of p53)
Which types of HPV are high risk
HPV 16 and 18
can lead to cervical cancer
sexually transmitted
How does HPV cause cancer?
Infects basal layer of cells
With persistent infection, integrates its DNA into host Genome
E6 protein binds to p53 and causes degradation
E7 binds to Rb (a tumor supressor which regulates the cell cycle (E2F TF which promotes cell cycle entry)
What preventative measures are there for HPV?
Gardasil vaccine
Protects against most dangerous forms of HPV and their cancer link
Also protects against more mild form, the formation of genital warts
Risks between diet and cancer
Obesity
30-40% increased cancer risk
Free radicals
DNA adducts
What is the significance of high fructose corn syrup?
Found in lots of high calorie foods now
Very cheap and abundant - lobbyists and corn subsidies
Was a part of cars for a little, then limited
Fructose
Vegetables and fruits have ~15g
These typically have other fiber, vitamins, minerals
Why is Fructose / sugar bad for us?
Normally: Glucose in blood stream, insulin released to aid glucose absorption, glucose is uptaken by muscles and brain and/or converted to glycogen
Here: Fructose goes through this whole process without being recognized, goes directly into liver
Most changes to fat / adipose tissue
Why is fat bad / does it increase cancer risk
Adipose tissue releases Leptin after you eat, makes you feel full
Leptin works on your hypothalamus, expenditure vs intake signal
Fat cells empty = no leptin, eat
Too many fat cells builds leptin resistance, always hungry (Leptin resistance essentially means no productive leptin signal) (“Gas gauge broken”
Describe the Princeton experiment
Rats being fed different diets
Those with access to high fructose corn syrup gained 48% more weight
Boy that was always hungry and tired, he was born with mutation affecting leptin
Gave him a leptin supplement, went to normal body size
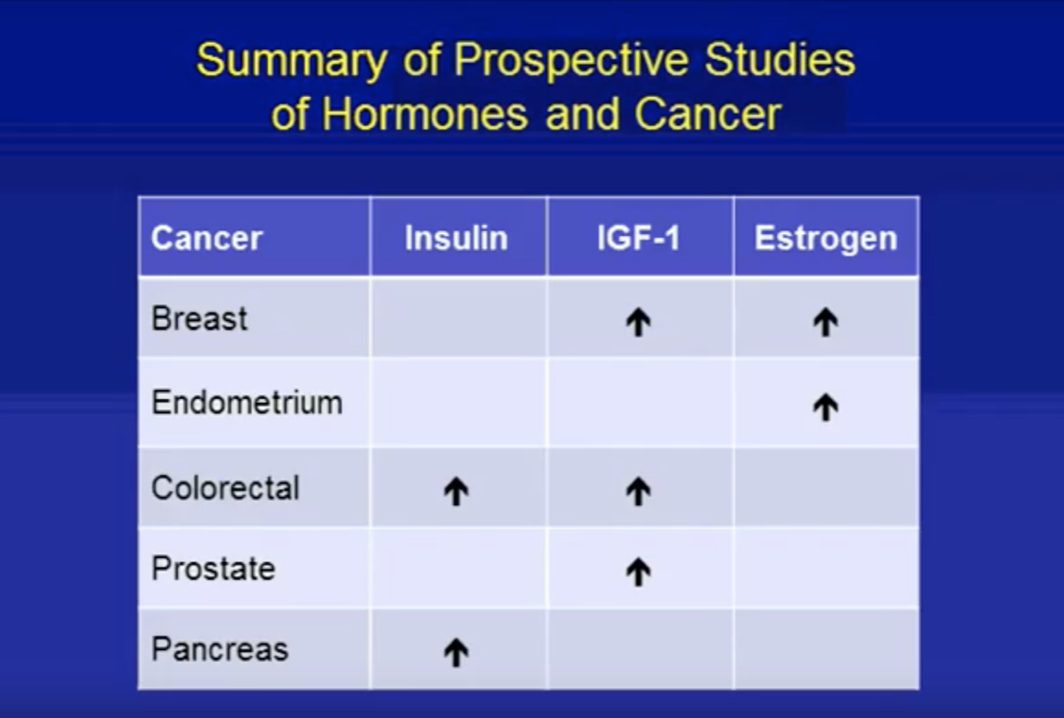
How does Obesity increase cancer risk
Higher sex hormone production
^ IGF1
^ Progesterone
^ Estrogen
v Apoptosis
^ Cell proliferation
^ levels of leptin, lower adepokines
By default, obese people have higher free radical count
Increased inflammation caused by these hormones and cytokines / interlukens
This results in the generation of free radicals
Higher insulin resistance
Obesity/fat cells → Inflammation → Free radicals → Oxidative stress
Estrogens
Steroid ring system
Estradiol
Estrone
Estrogen broken down, estrone is a hyper free radical due to its oxygen
Estrogen effects on cancer
Estrogen can increase the chances of developing a new spontaneous mutation, stimulate division of existing mutant and uterine cells
Higher estrogen = higher cell proliferation
Lower estrogen = cell death
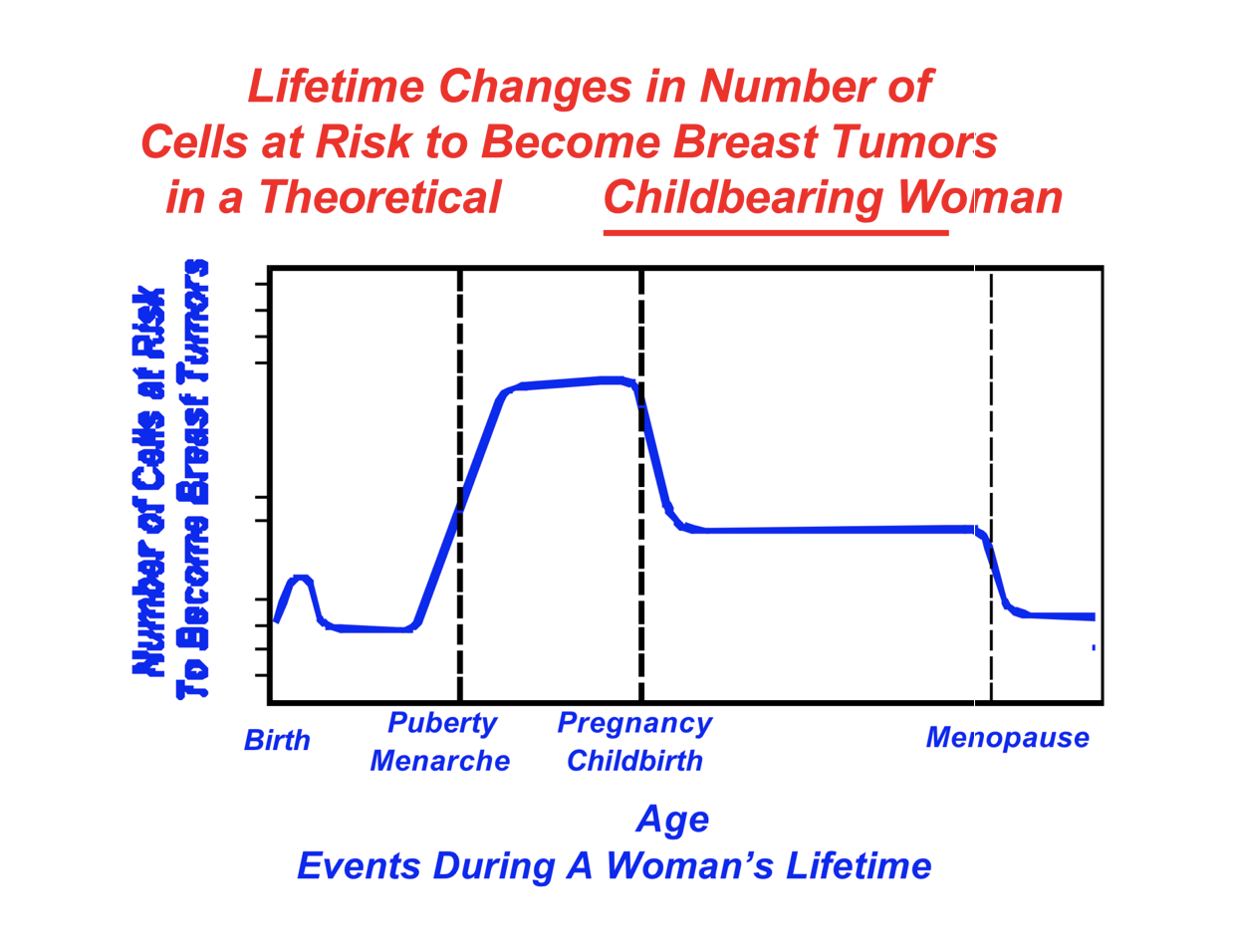
What is the function of estrogen? How does having children and getting a period earlier effect women (and their cancer risk)
Estrogen is a hormone found at higher levels in women
Controls cholesterol
Strengthens bones
Programs milk production
Prepares for fetus
Pregnancy and childbirth drastically lower estrogen levels in women
Lowest after menopause
Higher estrogen levels (from earlier period OR no childbirth) increase cancer risk
Cooking and cancer risk
Heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs) formed when meats are cooked at high temperatures
Exposure to high levels of HCAs and PAHs can cause cancer in animals; unclear if same for humans
What chemical adducts do carcinogens HCA and PAH cause
Benzopyrene
Estrone
3-methylcholanthrene
Activation of carcinogens
Many important carcinogens require chemical activation
Multiple enzyme systems involved in activation
Linkage from
Chemical activation
Adducts
Specific mutations
Cancer
What are bulky adducts?
Large, covalently attached chemical groups that form when carcinogens bind to DNA
Usually attach to guanine, lead to mutations if not repaired properly
Specifically, can cause problems in p53, leading to mutation
Describe what the differences are between human and carnivore intestines and how this may cause issues
Humans - Longer and less acidic
Carnivores - Very acidic and short
Issues:
if humans eat like obligate carnivores
Low fiber increases colon cancer risk
Fiber fuels gut bacteria, reduces inflamation
Whole grains, fruits, vegetables
High protein intake strains kidneys (urea)
Antioxidants
Remove excess free radicals
Colorful vegetables very high in them
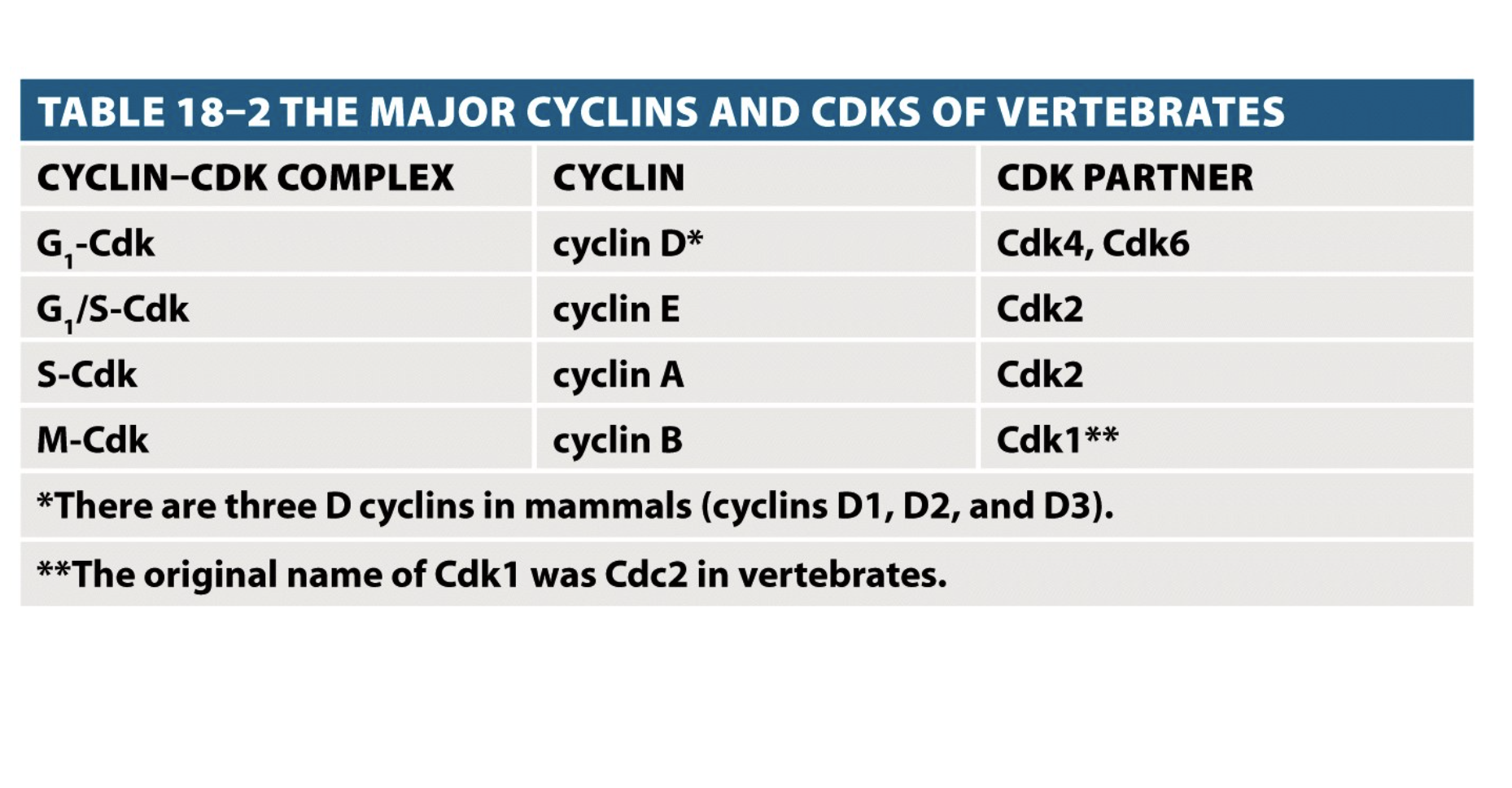
Cyclins and cdks
S-Cdk:
Initiate and complete DNA replication.
Prevent DNA replication during mitosis.
M-Cdk:
Induces assembly of the mitotic spindles and insure that the replicated chromosomes attach to the spindles.
Chromosomes condensation, microtubules rearrangement, nuclear envelope breakdown and reorganization of the Golgi and ER.
Cyclin conc rises gradually during interphase and then drops sharply after mitosis
R point and regulation
R point = restriction point
Cyclin D binds and activates CDK 4/6, this complex then begins to phosphorylate the Rb protein
In its active, unphosphorylated form, Rb binds to E2F (TF), prevents them from innitiating replication stuff
Phosphorylation of Rb inactivates it → causes Rb to release E2F.
E2F further induces transcription of Cyclin E, which binds to CDK 2 to hyperphosphorylate the Rb protein, this is a positive feedback loop triggering the
Phosphorylation and dephosphorylation of CDK-cyclins
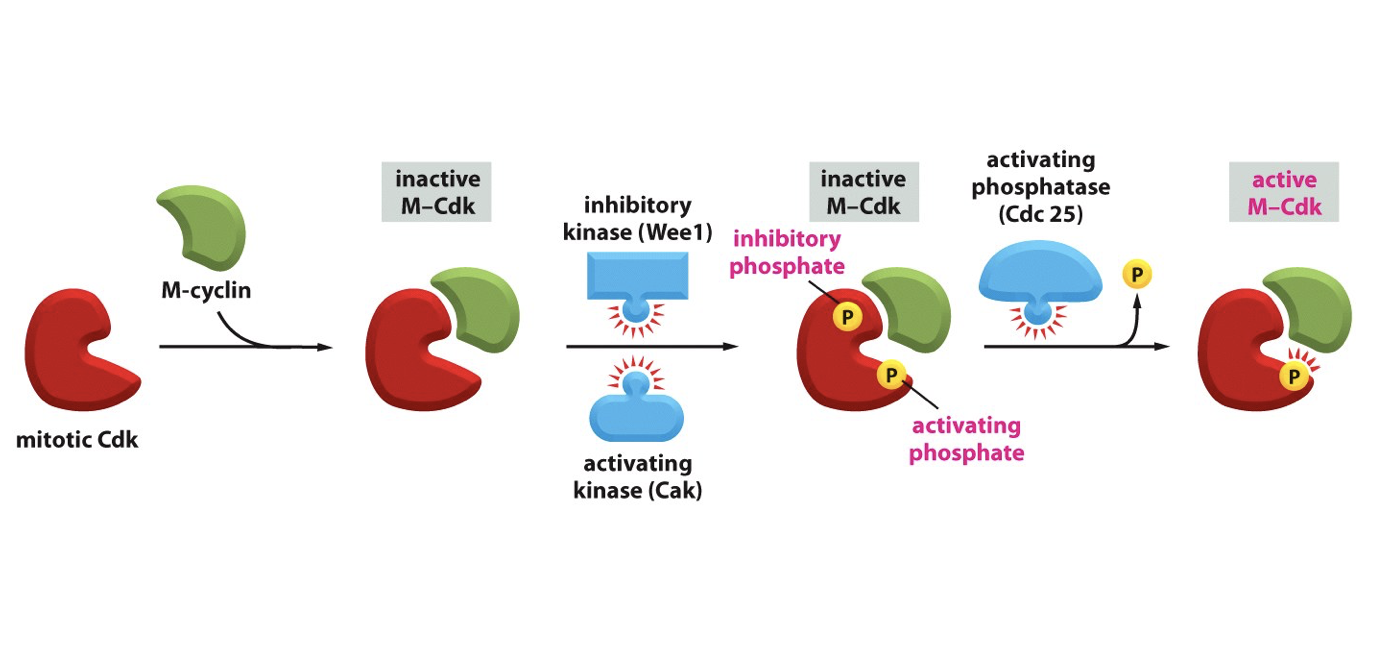
M-cyclin binds to M-cdk, inhibiting it
inhibitory kinase (Wee1) and activating kinase (Cak) bind phosphate to it, so there is both an activating and inhibiting phosphate on (= innactive)
activating phosphatase (Cdc25) removes the inhibitory phosphate, activating the m-cdk
Active M-Cdk exhibits positive feedback on Cdc25 phosphatase, inhibiting it
Cyclin E and breast cancer progression
High total levels of Cyclin E = More death chance
EGFR
Epidermal Growth Factor Receptor
Receptor for EGF
Mutations to EGFR are highly linked to cancer
Three domains - Extracellular, Transmembrane, intracellular domain (Tyrosine kinase)
Ligand (EGF) binds and conforms EGFR, phosphorylates a tyrosine initiating a signaling pathway
Tyrosine at 590, 700, 1060 aa position
EGFR pathway
Grb 2 (protein) binds to phosphorylated tyrosine on the receptor, recruits SOS binds through the SH2 and SH3 domains of the Grb 2 protein
SOS binds to Ras (proto-oncogene) which further signals (this is the MAP kinase pathway (Ras→)
recruiting Raf1
which recruits Mek
Recruits Erk1/2 (= MAPK)
—>Proliferation
Phospholipids and phospholipases in signal transduction
1) Phosphatidyl-inositol (PI):
OH groups on inositol phosphorylated
2) Phosphatidyl-inositol diphosphate (PIP2)
PI3K (kinase)
3) PIP3
PTEN can convert PIP3 back to PIP2
AKT / PKB binds to PIP3
Hyperactivity of PIP3 or inactivity of PTEN causes cancer
PI3K mutation leads to much higher death rates
Role of AKT / PKB (protein kinase B) in signaling pathways
Involved with MDM2, which degrades P53
Can go back and turn on Raf1, which can turn on MAPK pathway
Cell cycle checkpoints
G1 Checkpoint - Enter mitosis—are conditions favorable?
G2 Checkpoint - Is all DNA replicated? Is all DNA damage repaired?
M phase checkpoint - Are all chromosomes atrached to the spindle properly
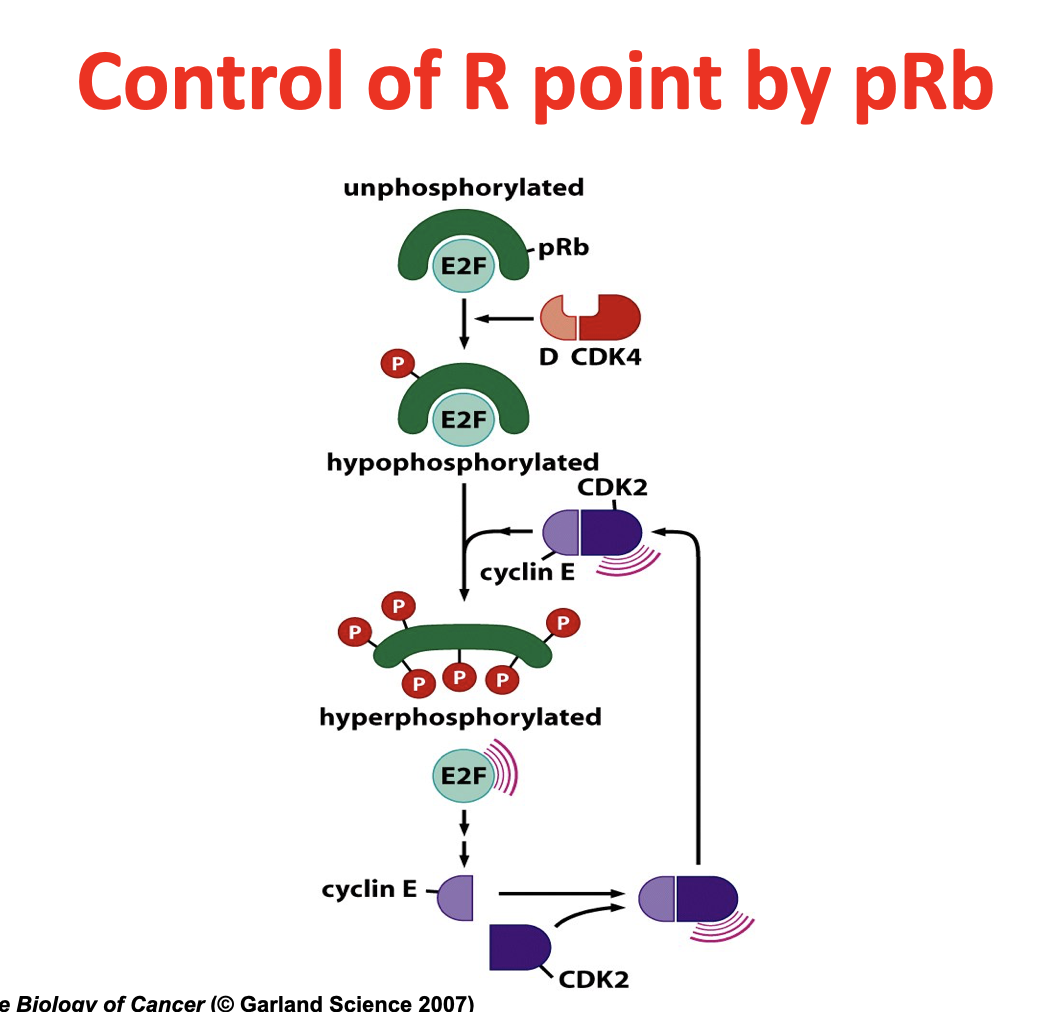
Control of R point by pRb
Message comes in, Cyclin D and CDK4/6 bind together and hypophosphorylate Rb
Normally Rb holds on to E2F
Rb gets hyperphosphorylated by CDK2 and Cyclin E (once they accumulate)
E2F released and generates more Cyclin E and CDK2, positive feedback
Passes the R point, once crossing it cannot go back
Inhibitors of Cyclins
D-CDK 4/6
P16 INK4A
P15 INK4B
P18 INK4C
P19 INK4D
Inhibitors of other cyclins
P57 Kip2
P27 Kip1
P21Cip1
Result of having higher levels of cyclin E
Poorer prognosis with breast cancer
Hallmarks of Apoptosis
1) Plasma membrane herniated and form structures called blebs
2) Shrinkage of cell
3) Collapse of nucleus and fragmentation of genomic DNA
4) Fragmentation of golgi apparatus and cleavage of nuclear protein called PARP
5) Breakage of cells and formation of membrane-bound apoptotic bodies
6) Rapid phagocytosis by macrophages or neighboring cells
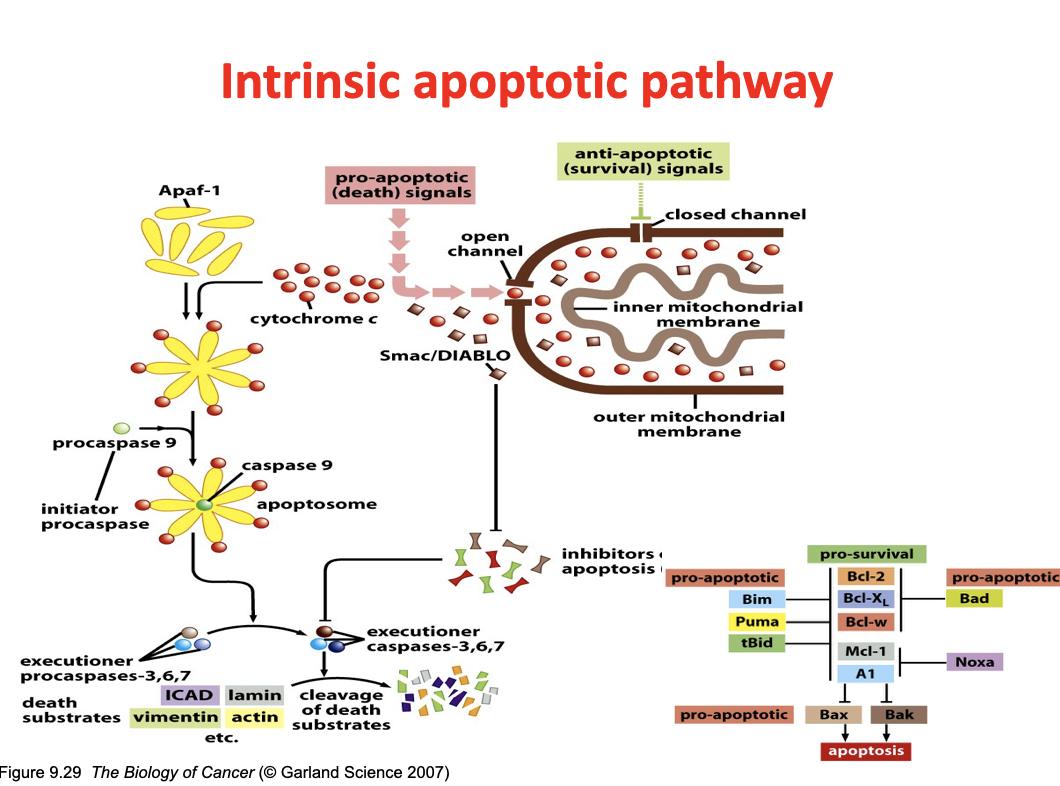
Apoptosis cell signaling
Cytochrome c binds to Apaf-1
These form an apoptosome
Recruitment of the initiator procaspase procaspase-9 (turns to active caspase-9)
This activates executioner caspases (procaspase 3, 6, 7—>caspase 3, 6, 7)
Executioner caspases cleave proteins like lamin, actin, vimentin, etc (known as death substrates)
Smac/diablo is released from mitochondria in response to apoptotic signals (in addition to cytochrome c)
Inhibits IAPs (inhibitor of apoptosis proteins) which normally inhibit the caspases
Activation of apoptosis by p53
With damage, ATM/ATR kinases phosphorylate p53
Bax - forms holes in the mitochondrial membrane releasing cytochrome c and SMAC/Diablo
FAS also turned on by p53
IGFBP-3 released from cell, binds IGF, which binds to receptor turning on PI3K
Roles of angiogenesis
Forms new blood vessels during healing at site of injury
Develops collateral circulations during ischemia
Allows tumors to grow
Pathogenic vessel growth can result in diseases
Angiogenesis signaling
VEGFR family
VEGFR-A most prevelant/potent
VEGF-A binds to VEGFR-2 and other signals, allowing angiogenesis
Bevacizumab is a powerful treatment for anti-angiogenic activity
Pro and anti apoptotic genes
Pro
Bad
Bax
Bak
Puma
Anti
BCl2
BClx
MCl1
Tumor grading (TNM)
T - Tumor (size, cm)
N - Nodes (Regional metastases)
M - Metastases (distant)
Stages I-IV
Stage I = Local disease, no nodal or distant metastases
Stage IV - Distant metastases
Imaging procedures
X-rays:
X-rays use low doses of radiation to create pictures of the inside of your body.
CT Scan:
An x-ray machine linked to a computer takes a series of detailed pictures of your organs..
Contrast CT Scan:
material helps make these pictures easier to read.
Nuclear scan:
For this scan, you receive an injection of a small amount of radioactive material, which is sometimes called a tracer. radioactive substance quickly. This type of scan may also be called radionuclide scan.
Ultrasound:
An ultrasound device sends out sound waves that people cannot hear. The waves bounce off tissues inside your body like an echo. A computer uses these echoes to create a picture of areas inside your body. This picture is called a sonogram.
MRI:
A strong magnet linked to a computer
PET scan:
For this scan, you receive an injection of a tracer. Then, a machine makes 3-D pictures that show where the tracer collects in the body. These scans show how organs and tissues are working.
Diagnosis and staging of breast cancer
IDK look up a video or something
0 - 4 with 0 being non-invasive ductal carcinoma in situ
Cancer treatments
Chemotherapy
Alkylating agents to cause apoptosis (binds to guanine)
Antimetabolites: Interfere with DNA synthesis
5-florauracil
methotrexate
Hormone therapy - Estrogen or progesterone
Treat with tamoxifen: competes for receptors and does not allow estrogen or progesterone to bind
Antibiotics - Interfere with enzymes involved in DNA replication
Doxorubicin
Adriamycin
Cancer related proteins / receptors and their treatments / inhibitors
VEGF (angiogenic protein): Bevacizumab
EGFR: Gefitinib, Erlotinib
EGF: Trastuzumab, Pertuzumab
VEGFR: Sunitinib
PDL-1 and PD1
T cells become activated upon recognizing a foreign or tumor antigen via their T-cell receptor (TCR).
Tumor cells or APCs express PD-L1.
PD-L1 binds to PD-1 on T cells.
This sends an inhibitory signal into the T cell:
↓ T cell proliferation
↓ Cytokine production
↓ Cytotoxic activity
Result: Immune response is suppressed.
In Cancer:
Many tumors overexpress PD-L1 to "turn off" nearby T cells.
This allows tumors to evade immune detection and destruction.