Absorption of Mineral Salts
Absorption of mineral salts:
A) Active Absorption: i) Cytochrome Pump/Lundegardh theory
ii) Proton-anion Co-transport
iii) Lecithin Carrier Concept
B) Passive Absorption: i) Simple diffusion theory
ii) Ion Exchange theory
iii) Donan Equilibrium
iv) Mass Flow theory
ACTIVE ABSORPTION
Mnemonic: 3RACHA
3: Three theories
R: Respiration rate increase
A: Active
C: Carrier ion/molecule
Cation
Cytochrome pump theory
Co-transport theory
Carrier concept
H
A: Energy
Enzyme
CYTOCHROME PUMP THEORY
Cyto→ cell
chrome→ color/Pigment (Fe²+ & Fe³+)
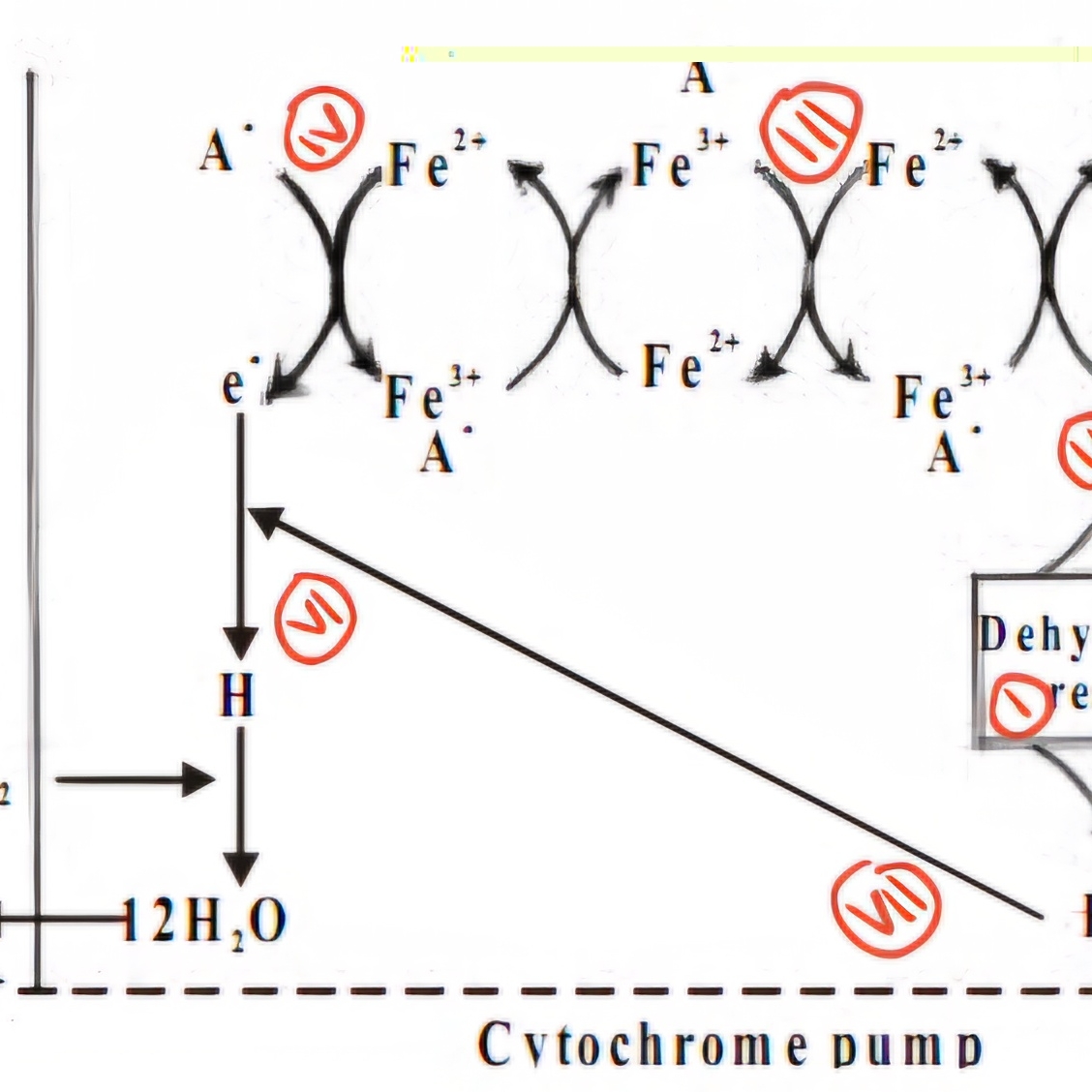
1. Start with dehydrogenase (Step I)
•Inside the cell membrane, the dehydrogenase enzyme releases protons (H⁺) and electrons (e⁻) from a molecule.
•This basically means a molecule is losing electrons = oxidation is happening.
2. Electrons enter the cytochrome chain (Step II)
•These electrons travel through a set of proteins called the cytochrome chain (part of the ETC).
•Think of cytochromes as “electron carriers” that pass electrons like a relay race.
3. Iron (Fe) gets involved (Step III & IV)
•The cytochromes contain iron (Fe), which switches between two states:
•Fe³⁺ (Ferric, oxidized state) → Fe²⁺ (Ferrous, reduced state) when it gains an e⁻.
•Fe²⁺ → Fe³⁺ when it loses an e⁻.
4. Electrons + Oxygen = Water Formation (Step V & VI)
•On the outer surface, Fe²⁺ donates its electron to oxygen (O₂).
•Oxygen now absorbs an anion (A⁻) to balance the charge.
•Electrons, protons (H⁺), and oxygen come together to form water (H₂O).
5. Anion (A⁻) moves back (Step VII)
•The anion (A⁻) returns inside the membrane through the cytochrome system.
6. Cations move passively (Step VIII)
•To balance things out, a cation moves passively into the cell (probably H⁺ or another ion).
•This helps maintain the electrochemical gradient, which is crucial for ATP production.
Trick: Cytochrome as the electron Carrier that pass electrons like a relay race
●This whole system is about moving electrons and protons to help form energy.
●Oxygen is the final electron acceptor, and in the end, water is formed as a byproduct.
PROTON ANION CO-TRANSPORT
Proton→ H+
Anion→ A-
Co-transport→ together
It’s basically about protons (H⁺) and anions (A⁻) moving together across a membrane. This helps the cell maintain balance and generate energy.
1. Proton Pumping (Step 1)
•The cell uses ATP (energy) to actively pump H⁺ ions out of the cell through protein carriers in the membrane.
•This means the inside of the cell loses protons and becomes more negatively charged compared to the outside.
2. Proton Motive Force (PMF) (Step 2)
•Because we pumped H⁺ out, two gradients are created:
•pH Gradient: Outside is more acidic (more H⁺), inside is less acidic.
•Charge Gradient: Outside is positive, inside is negative.
•The cell now wants to bring H⁺ back in to fix this imbalance.
3. Proton Movement Back (Step 3)
•The cell doesn’t waste energy; it lets H⁺ flow back inside using special proton carriers in the membrane.
•This movement is passive (doesn’t use energy, like rolling downhill).
4. Anion Movement (Step 4)
•Since positive charges (H⁺) are coming in, negative ions (A⁻) move in along with them to neutralize the charge.
•This helps keep the electrical balance in the cell.
5. Co-transport (Step 5)
•Because H⁺ and A⁻ move together, this whole process is called proton-anion co-transport.
Trick: You and your bestie (H⁺ & A⁻) are at a concert. You need to get to the front, but security won’t let you in alone. So, you sneak in together by blending into the crowd.
It’s like a buddy system: one can’t move alone, so they go together.
LECITHIN CARRIER CONCEPT
Lecithin→ a type of lipid, acts as a carrier for ions across the cell membrane
1. Lecithin binds with ions:
•It grabs onto positively charged ions (cations, like Na⁺ or K⁺) and negatively charged ions (anions, like Cl⁻) to form a temporary complex.
2. Lecithin transports the ions:
•This complex moves across the cell membrane, carrying the ions with it.
3. Ions are released inside the cell:
•Once it reaches the other side of the membrane, lecithin releases the ions where they’re needed.
4. Separation of cation & anion:
•Lecithin contains choline-phosphatidic acid, which helps in separating the cation and anion after transport.
Trick: Think of lecithin as a delivery truck: It loads up ions (packages) and carries them across the cell membrane (the highway). Once it reaches the destination, it unloads the ions and returns for more deliveries.
PASSIVE ABSORPTION
Mnemonic: No DIM
No: Normal Respiration rate
No Absorption
No carriers
No energy
No enzyme
D: Donnan Equilibrium
Diffusion theory
I: Ion Exchange theory
M: Mass Flow theory
SIMPLE DIFFUSION THEORY
•Ions move from high to low concentration.
•No carriers, no ATP, just pure chaotic spreading.
•Happens when soil has higher ion concentration than the root.
•Classic and boring, but solid.
Catchphrase: “High to low, and I don’t even try.”
ION EXCHANGE THEORY
•Ions in soil get swapped with ions from the root surface.
•Two main types:
•Contact exchange: Ions literally switch places between root surface and clay/soil particles.
•Carbonic acid exchange: CO₂ from root respiration forms carbonic acid → releases H⁺ → H⁺ swaps with soil ions.
Catchphrase: “You give me one, I give you one back.”
DONAN EQUILIBRIUM
•Root cells have fixed, non-diffusible ions (like proteins or organic acids) that attract oppositely charged ions from the outside.
•Those opposites sneak in through osmosis-like behavior.
•It’s like that quiet person in class who just attracts drama (ions) without even doing anything.
Catchphrase: “Attract & trap, no ATP cap.”
MASS FLOW THEORY
•Ions get dragged along with the flow of water during transpiration stream.
•The faster water flows (due to transpiration), the more ions get swept up.
•Think of it like leaf-induced water Uber pulling ions along for the ride.
Catchphrase: “Go with the flow—literally.”