Chemical and Physical Change, Chemical and Physical Change, Physical Properties & Chemical Properties
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Physical Change
Crushing A Can

Chemical Change
Burning Wood

Chemical Change
Baking A Cake

Physical Change
Melting A Snowman

Physical Change
Ripping Paper

Chemical Change
Growing A Plant

Chemical Change
Exploding Fireworks

Physical Change
Boiling Water

Physical Change
Breaking Glass

Physical Changes in Matter
Cleaning A Penny

Chemical Change
Mixing Baking Soda With Vinegar

Physical Change
Freezing Water

Physical Property
can be observed and measured without changing the kind of matter being studied

Melting Point
temperature at which a substance changes from a solid to a liquid
Boiling Point
temperature at which a substance changes from a liquid to a gas

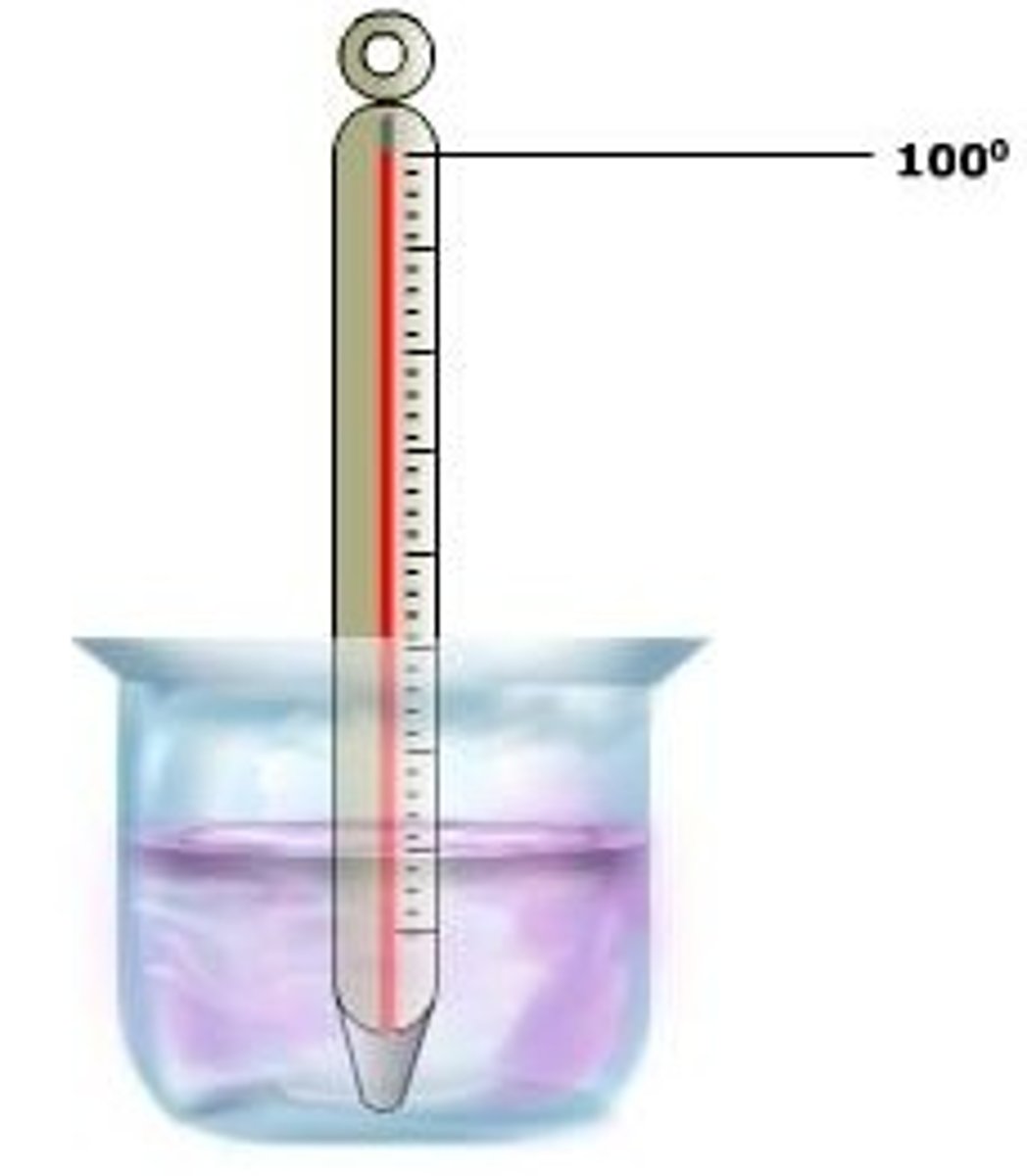
Boiling Point of Water
100 degrees celcius; 212 Fahrenheit
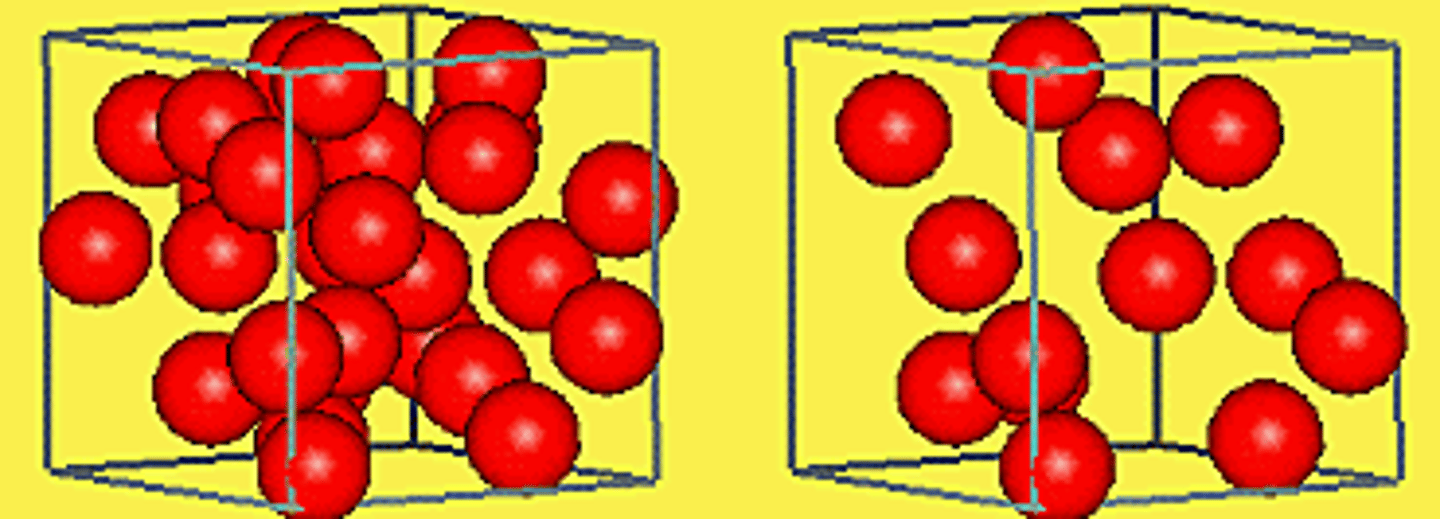
Density
the relationship between the mass of a material and its volume
Chemical Property
can be observed when substances undergo a change in composition; the ability to react (combine) with another substance

Flammability
A material's ability to burn in the presence of oxygen

Reactivity
The ease and speed with which an element combines, or reacts, with other elements and compounds.

Formula for Density
Density = mass/volume
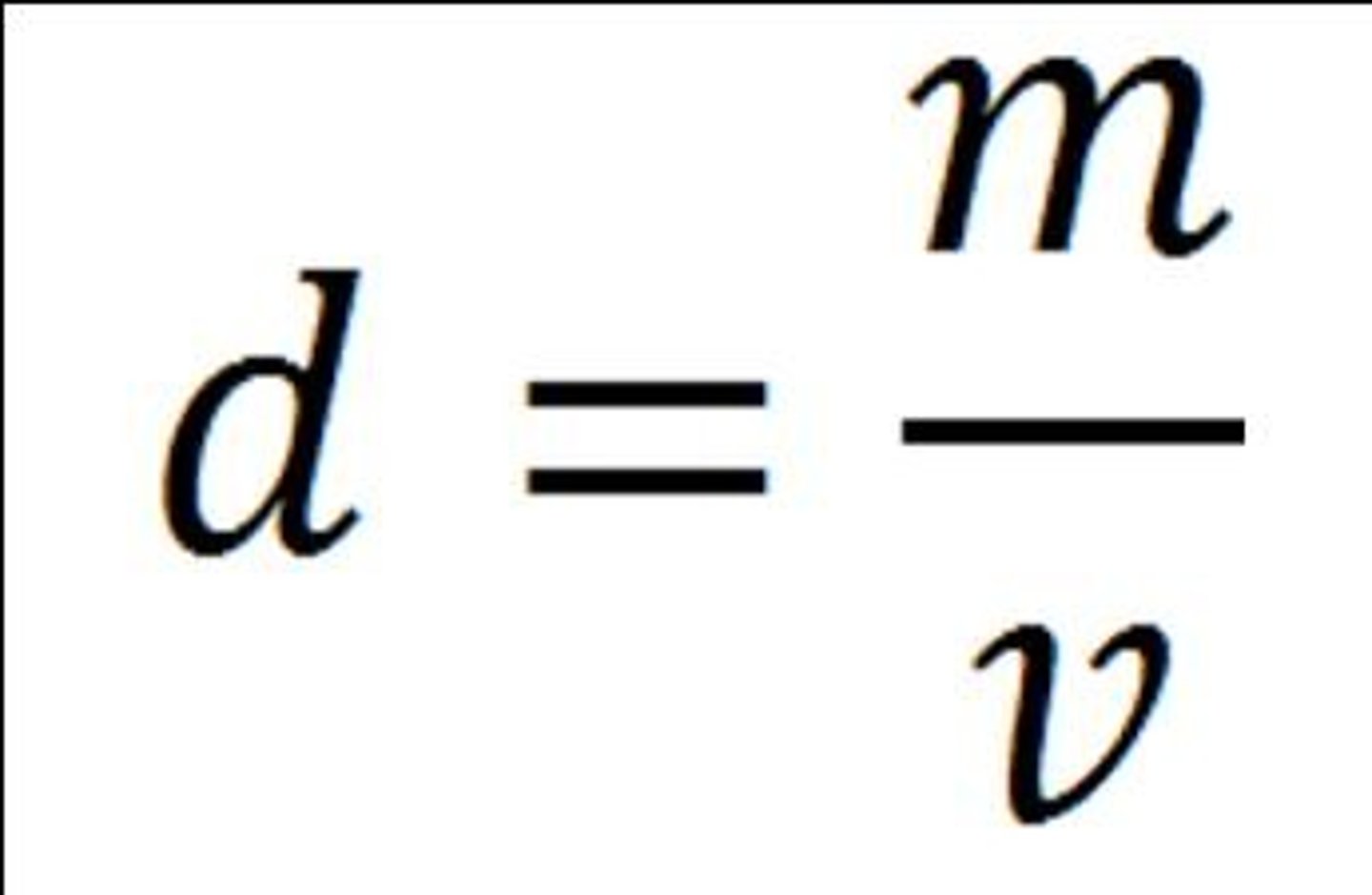
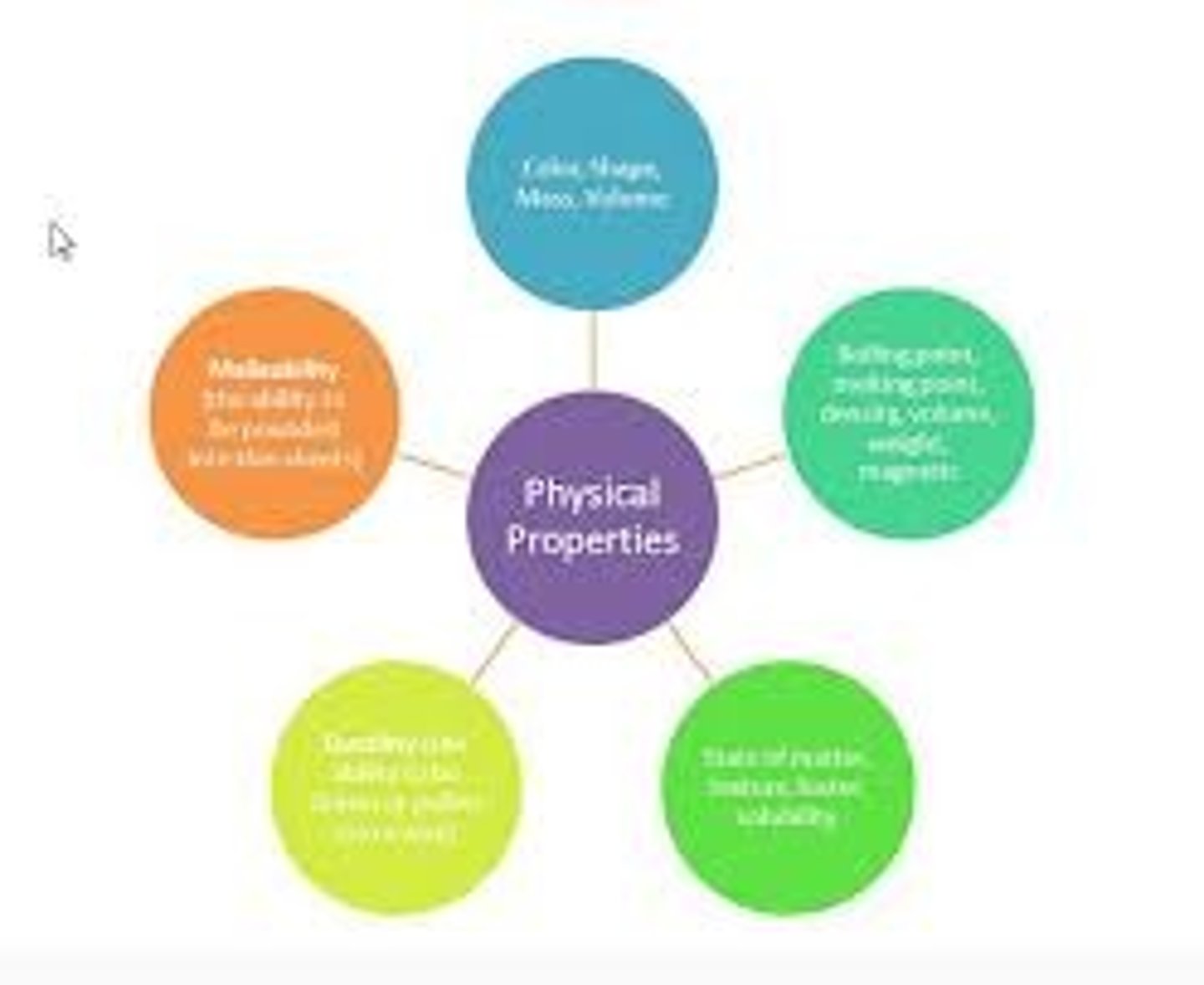
Physical Properties of Matter
Appearance changes: Melting Point; Boiling Point; Density; Color, Conductivity, Ductile
Malleable
Chemical Properties of Matter
reaction to other types of matter such as: flammability; reactivity

Conductivity
Ability to conduct heat or electricity
Ductile
Ability to be drawn out into a wire

Malleable
Ability to be flattened or spread out

Solubility
the physical property which describes how easily a substance dissolves or breaks down, and become liquid.

chemical reaction
the process by which one or more substances change to produce one or more different substances
Evidence of a chemical reaction
-production of gas
-formation of precipitate
-change in temperature
-change in color
-change in odor

Viscosity
A liquid's resistance to flowing