BIOM*3200 Midterm 2
1/313
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
314 Terms
claude bernard
father of modern physiology; said that our internal environment remains remarkably constant despite changes in the external milieu
walter cannon
coined the term homeostasis to describe the relative stability of the internal environment
components of homeostatic systems
negative feedback: shift in physiological variable outside normal range
sensor: detects shift
integration/control centre: sets normal range and coordinates the next set of events
response system: changes environment to get back to set point
blood pressure homeostasis
blood is evenly distributed throughout the body when lying flat
when standing, blood pools in legs
pooling blood causes reduction in venous return and therefore cardiac output, so pressure falls
drop in blood pressure is detected by baroreceptors in the aortic arch and carotid sinus
the baroreceptors respond by increasing sympathetic and reducing parasympathetic outflow
peripheral vascular resistance is increased, which causes venous return, cardiac output, and blood pressure to increase
major regulatory systems to maintain homeostasis
skin, cardiovascular, renal, digestive, respiratory, musculo-skeletal
major regulated factors for homeostasis
water, electrolytes/pH, nitrogenous compounds, oxygen, carbon dioxide, temperature, toxicants
endocrine dysfunctions
hyper-function: too much hormone
hypo-function: too little hormone
resistance: too little effect
endocrine gland
a tissue which releases a substance into the bloodstream; this substance then travels via the blood to influence a target cell
classic minkowsi experiment
surgically removed the pancreas in the dog; the dog developed symptoms of diabetes; implanting pieces of pancreas under the skin prevented symptoms of diabetes
banting and best
the discovery of insulin; identified antidiabetic substance in pancreatic extracts; injected extracts prevents symptoms of diabetes
insulin
peptide hormone produced by beta cells of the pancreas; promotes absorption of glucose from blood to skeletal muscle and fat tissue; stored in inactive form as a hexamer of zinc ions and histidine residues
what makes a chemical a hormone?
proteins and polypeptides; steroids (cholesterol derivatives), glycoproteins; amines (catecholamines or thyroid)
autocrine hormones
a cell responds to a signal it secreted
paracrine hormones
the hormone acts on a cell nearby; doesn’t enter the bloodstream
endocrine hormones
hormone is released into the blood by an endocrine gland to reach the target; long distance
mode of secretion of peptide hormones: synthesis, storage, release from cell, transport in blood, half life, example
synthesis: in advance
storage: secretory vesicles
release from cell: exocytosis
transport in blood: dissolved in plasma
half life: short
example: insulin
mode of secretion of steroid hormones: synthesis, release from cell, transport in blood, half life, example
synthesis: on demand
release from cell: diffusion
transport in blood: bound to carrier proteins
half life: long
example: estrogen, androgen
mode of secretion of catecholamine hormones: synthesis, storage, release from cell, transport in blood, half life, example
synthesis: in advance
storage: secretory vesicles
release from cell: exocytosis
transport in blood: dissolved in plasma
half life: short
example: epinephrine, norepinephrine
mode of secretion of amine thyroid hormones: synthesis, storage, release from cell, transport in blood, half life, example
synthesis: in advance
storage: secretory vesicles
release from cell: diffusion
transport in blood: bound to carrier proteins
half life: long
example: thyroxine T4
overview of receptor binding
hormones bind to receptors in target cells; very high specificity for a particular hormone; continuous turn-over of receptor-hormone complex; receptors for most hormones are found in the plasma membrane of target cells; receptors for thyroid and steroid hormones are inside the target cells
transmembrane receptors
hormone binds to the extracellular domain of the receptor and activates one or more cytoplasmic signaling pathways, usually involving phosphorylation and enzyme activation; some lead to gene expression responses, others have local effect in target cell
adenylate cyclase pathway
hormone binds to receptor and the G-proteins dissociate
alpha subunit activates AC
catalyzes product of cAMP
removes regulatory unit from PKA
PKA activates other molecules for hormonal response
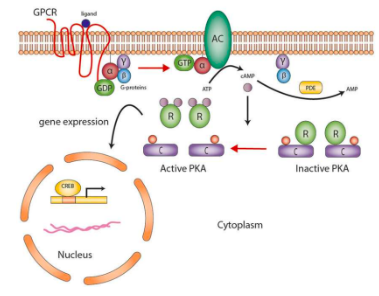
epinephrine and adenylate cyclase
epinephrine binds to B-adrenergic receptor on liver cell
G-proteins activated, subunit carrying GDP dissociates, GTP binds
subunit activates adenylyl cyclase which catalyzes ATP to cAMP
cAMP activates PKA, which activates phosphorylase
phosphorylase converts glycogen to G6P
G6P is turned into glucose in the liver
robert lefkowitz and brian kobilka
received nobel prize by determining how GPCRs work to understand network of signaling between cells; how cells sense their environments
phospholipase C-Ca2+ pathways
hormone binds to receptor, G proteins dissociate
activates phospholipase C (PLC)
causes breakdown of membrane phospholipid to IP3
IP3 binds to endoplasmic reticulum
release of stored Ca2+ into cytoplasm
Ca2+ activates other molecules
alpha vs. beta adrenergic receptors
alpha-adrenergic receptors activate phospholipase C via Gq; beta-adrenergic receptors activate adenylate cyclase via Gs
steroid hormone receptors process when hormone binds
steroid hormone transported bound to plasma carrier protein
steroid hormone binds cell cytoplasm receptor
translocates to nucleus, binds to DNA
acts as a TF to stimulate gene transcription
protein products produce response
thyroid hormone receptor process when hormone binds
thyroxine (T4) binds to carrier binding protein
T4 is converted to triiodothyronine (T3)
T3 uses binding proteins to enter nucleus
hormone-receptor complex binds DNA
new mRNA and proteins produced
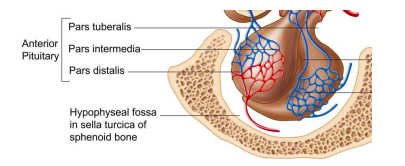
pituitary gland
physically connected to hypothalamus through an infundibulum (stalk); whole gland protected by bone; anterior pituitary (adenohypophysis) acts as an endocrine gland, posterior pituitary (neurohypophysis) acts as extension of neural tissue
hypothalamic hormones/factors
dopamine, prolactin releasing hormone, thyrotropin-releasing hormone, corticotropin-releasing hormone, somatostatin, growth hormone releasing hormone, gonadotropin-releasing hormone
dopamine
PIH; inhibits secretion of prolactin
prolactin releasing hormone (PRH)
stimulates release of prolactin
thyrotropin-releasing hormone (TRH)
regulates secretion of thyroid stimulating hormone (TSH)
corticotropin-releasing hormone (CRH)
regulates secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary
somatostatin
GHIH; inhibits secretion of growth hormone
gonadotropin-releasing hormone (GnRH)
regulates secretion of gonadotropin-releasing hormones, luteinizing hormone (LH), and follicle stimulating hormone (FSH)
CRH synthesis and inhibition
central stimulatory control: noradrenergic; stimulates pre-proCRH gene and protein expression (196 AA), processed to CRH (41 AA); stimulates pulsatile release of CRH
inhibitory influences: physiological levels of cortisol inhibit release of CRH
CRH production and release
produced by parvocellular neuroendocrine cells within paraventricular nucleus of hypothalamus; released at median eminence (base of brain) from neurosecretory nerve terminals, into blood vessels in the hypothalamic-pituitary portal systems
hypothalamic-pituitary-adrenal (HPA) axis
controls the body’s stress response
in response to stress, the hypothalamus releases CRH into bloodstream
CRH stimulates the anterior pituitary corticotropes to release ACTH and produce POMC
ACTH activates the adrenal cortex to produce glucocorticoids such as cortisol
glucocorticoids regulate activity of HPA axis through negative feedback
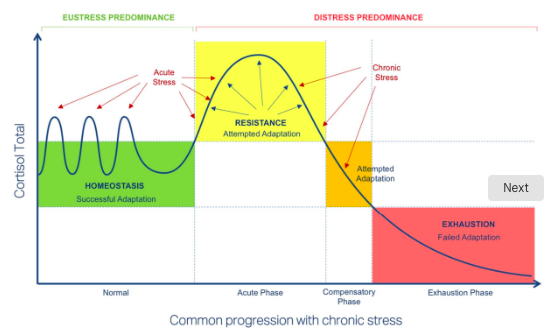
phases of HPA axis dysregulation
acute phase: after acute stress, there is resistance and attempted adaptation
compensatory phase: as chronic stress predominates, there is attempted adaptation
exhaustion phase: failed adaptation
the adrenal glands
made up of two embryologically distinct tissues that merged during development; adrenal cortex and adrenal medulla
adrenal cortex
secretes steroids/corticosteroids; glucosteroids controlled by ACTH, mineralocorticoids (eg. aldosterone) controlled by renin-angiotensin system; sex steroids controlled by ACTH
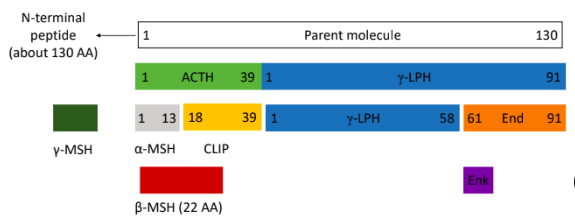
proopiomelanocortin (POMC) family
ACTH: adrenocorticotropic hormone; regulates adrenal cortex function
MSH: melanocyte stimulating hormone; skin pigmentation in response to UV radiation
End: B-endorphin; analgesic roles in CNS
Enk: enkephalin; analgesic roles in fetus
MC3,4,5- receptors: hypothermia, hypotension, feeding behaviour, appetite
convertases
enzymes that cleave POMC; different convertases give rise to different products
adrenal gland structure: outside layers to in and hormones produced
capsule; zona glomerulosa produces mineralcorticoids; zona fasciculata produces glucocorticoids; zona reticularis produces androgens; medulla produces epinephrine
functions of cortisol
protects against hypoglycemia; promotes gluconeogenesis to increase blood sugar; suppresses immune system and regulates inflammatory response; causes breakdown of skeletal muscle for gluconeogenesis; causes bone catabolism; affects brain function (mood, memory, learning)
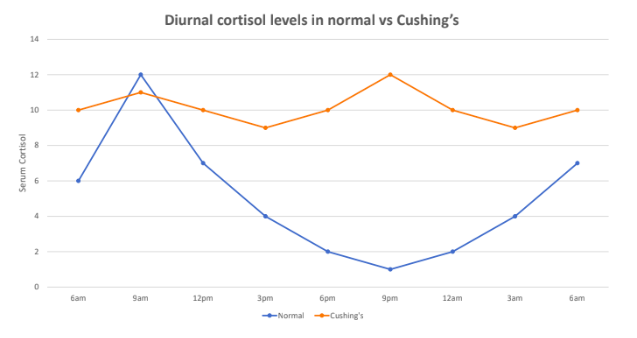
cushing’s syndrome
primary hypercortisolism; prolonged exposure to high levels of cortisol; can be caused by taking glucocorticoid drugs or by diseases that result in excess cortisol, ACTH, or CRH; causes changes in carbohydrate and protein metabolism, hyperglycemia, hypertension, muscular weakness; metabolic problems give rise to puffy appearance, CNS disorders such as depression, decreased learning and memory
cushing’s disease
secondary hypercortisolism; pituitary-dependent; a tumour in the pituitary gland produces large amounts of ACTH, causing adrenals to make excess cortisol
treatment for cushing’s disease and syndrome
surgery to remove pituitary or adrenal gland; medical management of signs and symptoms (insulin for diabetes, anti-hypertensives for BP); if not treated, disease worsens and overall health deteriorates and could lead to stroke or myocardial infarction
addison’s disease
primary hypocortisolism; adrenal insufficiency; many causes, may be from genetics, autoimmune destruction of adrenal cortex; can be acquired due to high-dose steroids given for >1 week which suppress CRH and ACTH and adrenal glands; symptoms include hair loss, blurry vision, abdominal pain, decreased appetite, darkening of skin, shaking, tremors, depression
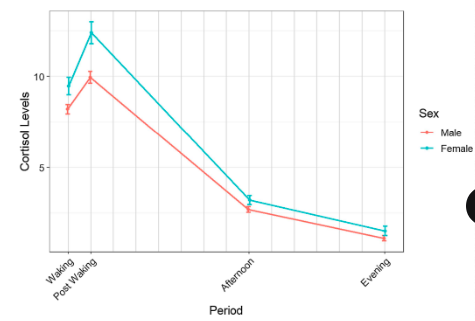
secretion patterns of adrenal cortisol
continuous, pulsatile, circadian release; peaks at post-waking; lowest in the evening
pituitary pars intermedia dysfunction (PPID)
affects older horses but has been diagnosed as young as 10 years old; caused by impaired pituitary (hyperplasia and hypertrophy of pars intermedia); leads to increased secretion of cortisol by adrenal glands; results in high blood glucose and suppression of immune system
common signs of pituitary pars intermedia dysfunction (PPID)
hypertrichosis (excessive hair growth); patches of long hair on legs, wavy hair on neck, changes in coat colour; muscle atrophy; excessive sweating; formation of fat pads on top of neck, tail head, and above or around eyes; pot-bellied appearance
diagnosis and treatment of pituitary pars intermedia dysfunction (PPID)
diagnose by measuring basal ACTH and fasting insulin; treatment with medication that acts on pituitary gland to decrease circulating ACTH (eg. pergolide); manage with exercise, weight loss, limiting starch and sugar in diet
thyroid gland
just below larynx on either side of trachea; lateral to the first 3-8 tracheal rings; 2 lobes connected by isthmus which is fibrous in cows and horses, indistinct in dogs and cats; largest purely endocrine gland
thyroglobulin
long peptide chain with lots of tyrosine side chains; found in colloid; made by follicular cells; thyroid peroxidase enzyme helps attach iodide to tyrosine residues
thyroid hormone synthesis
iodide is brought from the blood into follicular cells by sodium-iodide transporter, then into colloid by pendrin (transporter)
TPO removes an electron from iodide to produce iodine
iodine binds tyrosine residues in thyroglobulin to produce MIT or DIT
enzymes in the colloid modify the structures of MIT and DIT, joining them to produce T3 and T4
upon stimulation from TSH, thyroglobulin is taken up by follicular cells and cut, separating T3 and T4
T3 and T4 secreted out to bloodstream bound to carrier protein
MIT and DIT production and modifications
attachment of 1 iodine on a tyrosine ring produces monoiodotyrosine
attachment of 2 iodines on a tyrosine ring produces diiodotyrosine
MIT + DIT produces triiodothyronine (T3)
DIT + DIT produces tetraiodothyronine (T4)

thyroid hormone transport
99% of thyroid hormones in blood circulation bound to plasma carrier protein thyroxin-binding globulin (TBG); some are biologically active and must lose carrier protein to elicit effects in target cells
circadian rhythm of thyroid hormones
secretion highest in humans between 10am and 2pm; elevate basal metabolic rate; secretion highest in rodents at night instead of during the day
patterns and regulation of TRH secretion
pulsed secretion from hypothalamus; young animals secrete more than older animals; stress and cold result in increased secretion
physiological action of thyroid hormones
elevate basal metabolic rate; normal gonadal development and function; normal embryonic/fetal development, particularly development of CNS; production impaired with age
hypothyroidism
abnormally low basal metabolic rate; weight gain, lethargy, intolerance to cold
hyperthyroidism
increased basal metabolic rate; weight loss, muscular weakness, nervousness, protruding eyes (exophtalmos)
causes of hypothyroidism and hyperthyroidism
insufficient dietary iodide; thyroid gland defect; impaired thyroid hormone pathway; insufficient TSH from anterior pituitary; insufficient TRH from hypothalamus; mutant TSH or TRH receptors (genetic); mutant TH transport proteins; autoimmunity
cretinism
congenital deficiency of thyroid hormones; usually due to innate maternal hypothyroidism or iodine deficiency; reduced physical growth and development delays; treatment with thyroxin (T4) soon after birth (<1 month of age) completely or almost completely restores mental development by age 5
terminal brain differentiation of thyroid hormones
thyroid hormone-dependent brain development begins in utero and is completed after birth; important for dendritic and axonal growth, myelin formation, synapse formation, neuronal migration; maternal thyroid hormones first supply needs of the embryo/fetus
goiters pathophysiology
abnormal thyroid growth due to hypothyroidism
low iodide intake results in low thyroid hormone production
low plasma thyroid hormones results in high TRH
high TRH results in high plasma TSH
high plasma TSH stimulates excess growth of thyroid
graves disease
due to hyperthyroidism; autoimmune antibodies activate thyroid gland, leading to high plasma T3 and T4; high thyroid hormone concentration leads to low TRH and low plasma TSH; exophthalmia (protruding eyes)
symptoms of hyperthyroidism in cats
weight loss despite increased appetite; increased thirst; increased urination; increased heart rate; vomiting; diarrhea; restlessness; hyperactivity; greasy or matted hair
primary hypothyroidism in dogs
usually from destruction of thyroid; lymphocytic thyroiditis (immune infiltration) affects 50% doberman pinschers; idiopathic atrophy of thyroid (thyroid tissue lost and replaced by adipose cells); predisposed breeds are doberman pinschers, golden retriever, irish setter, miniature schnauzer, dachshund, cocker spaniel, airedale terrier
secondary hypothyroidism in dogs
thyroid destruction secondary to neoplasia affects 40% of dogs with cancer
congenital hypothyroidism/cretinism/pituitary dwarfism
75% of both lobes must be non-functional before developing clinical signs
myxedema (swelling of tissues with severe hypothyroidism), stupor, coma
thyroid disorder treatments
surgery (hemithyroidectomy), hormone supplementation, radiation therapy (for cancer), blockers (thiouracil derivates and thiocarbamides decreases iodination and conversion from T4 to T3); stimulants (furosemide increases conversion from T4 to T3); diet, electrolyte infusions
natural vs. synthetic thyroid hormone medication
synthetic formulations contain only T4 which must be converted to T3 by the body for it to work; by the de-iodinase enzyme; may add cytomel (T3) in addition to synthroid (T4)
natural is preferred because synthetic lacks T2, T1, and calcitonin
supplementing with calcitonin helps
calcitonin production after total thyroidectomy
may be deficient or absent in patients because total thyroidectomy removes C cells (parafollicular cells)
locations of calcium
99% found in bones stored as hydroxyapatite made of calcium salts and phosphate provide structural integrity
1% in soft tissues: intracellular 0.9% and extracellular 0.1%; essential for several normal biochemical processes
extracellular calcium
very tightly regulated; 50% ionized; 40% protein-bound; 10% complexed with phosphate and citrate; in extracellular fluid and bone
intracellular plasma
very tightly regulated; more abundant than extracellular; largely associated with membranes in mitochondria, endoplasmic reticulum, plasma membrane
total body calcium formula
intake - output
intake from the diet: 1/3 absorbed in small intestine; absorption is hormone regulated; recommended 1000 mg/day
output: kidneys
3 hormones that regulate movement of calcium between bone, kidneys, and intestine
parathyroid hormone (PTH), calcitriol (vitamin D3), calcitonin
parathyroid hormone
a peptide made by parathyroid gland; secreted continuously; not stored; helps regulate calcium
parathyroid gland
essential for life; seen in terrestrial amphibians onwards; 2 cell types within
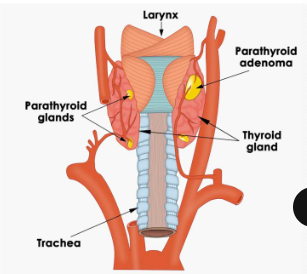
two cell types in the parathyroid gland
chief cells that produce PTH; oxyphils
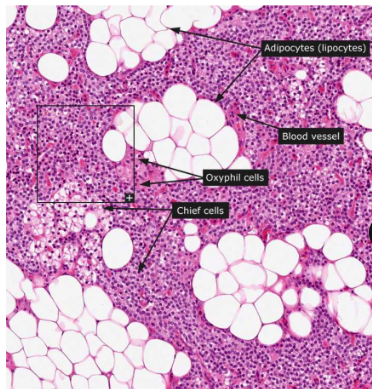
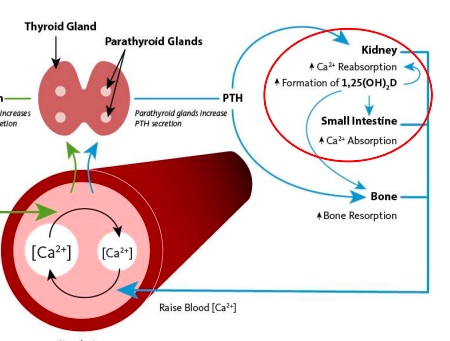
3 mechanisms to raise blood Ca2+ via PTH
stimulates osteoclasts to resorb bone
stimulates kidneys to resorb Ca2+
stimulates kidneys to produce enzyme needed to activate vitamin D, which promotes better absorption of Ca2+ from food/drink across intestinal epithelium
hypocalcaemia
low blood calcium; PTH secretion increases
hypercalcaemia
high blood calcium; decrease PTH secretion
bone deposition
by osteoblasts; secrete a matrix of collagen protein, which becomes hardened by deposits of hydroxyapatite
bone resorption
by osteoclasts; dissolves hydroxyapatite and return the bone Ca2+ and phosphate to the blood
calcium in bone; bone structure
bone is constantly formed and resorbed; contains calcified ECM that forms when calcium phosphate crystals precipitate and attach to a lattice support; calcium in bone is mainly in hydroxyapatite crystal form, but a small fraction is ionized and readily available
low blood calcium: process to bring back to normal
parathyroid gland increases PTH secretion; PTH stimulates kidneys to resorb calcium and produce 1-alpha-hydroxylase enzyme needed to activate vitamin D; vitamin D3 will act on intestines to absorb more calcium from food/drink; also stimulates bone resorption
vitamin D synthesis
vitamin D3 is produced from its precursor 7-dehydrocholesterol under the influence of UVB sunlight
inactive vitamin D3 secreted into blood from skin/intestine
goes to liver and has a hydroxyl group added to C25
requires hydroxyl group addition to C1 to become active; done by enzyme in kidneys stimulated by PTH)
vitamin D function
stimulates intestinal absorption of calcium; directly stimulates bone resorption by promoting formation of osteoclasts
sources of vitamin D
production of vitamin D in the skin; food sources (milk, eggs, fish); exposure to sunlight; people in northern/southern latitudes have to ingest it through diet or supplements
calcitonin
made in C cells of thyroid in response to high calcium; thought to only play a minor role in adult humans because thyroidectomy patients are not hypercalcaemic
high blood calcium: process to bring back to normal
thyroid gland increases calcitonin secretion
calcitonin acts on the kidney to stimulate excretion of calcium in urine
acts on the small intestine to decrease calcium absorption
inhibits osteoclast activity for less bone resorption
phosphate metabolism
controlled by the same mechanisms that regulate calcium metabolism to return or receive phosphate to/from bone, kidney filtrate, and GI tract
hyperparathyroidism
parathyroid too active; hypercalcaemia; increased bone resorption causing fractures; mineralization of soft tissues; increased thirst and urination because calcium blocks ADH effects
hypoparathyroidism
parathyroid not active enough; hypocalcaemia; muscular weakness; ataxia; cardiac arrhythmias
vitamin D deficiency
results in poor bone mineralization; in children, causes rickets with bone pain, stunted growth, deformities; in adults, causes osteomalacia with bone pain and fractures
osteoporosis
most common disorder of bone; reduction of bone quality due to excess absorption; risk of bone fractures; risk factors are sex (females after menopause), lack of exercise, calcium deficient diet