L4: Immune Cell Migration and Infiltration
1/43
Earn XP
Description and Tags
Flashcards covering immune cell migration, infiltration steps, key mediators (histamine, prostaglandins, chemokines, selectins, integrins), and the processes of physiologic and pathologic inflammation.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
44 Terms
What is one distinguishing property of immune cells compared to other tissue cells?
Their ability to migrate:
Can migrate from blood into tissues, be “homed” to these tissues & recirculate into the blood or to lymphoid tissues.
Immune responses: Combination of systemic and local events
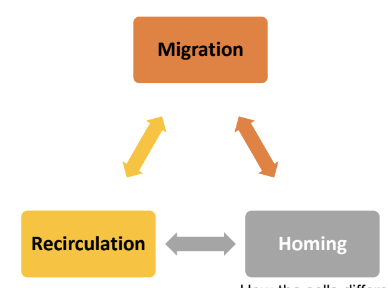
What are the three core concepts related to immune cell movement mentioned in the lecture?
Migration, Recirculation, and Homing
combination of systemic and local events
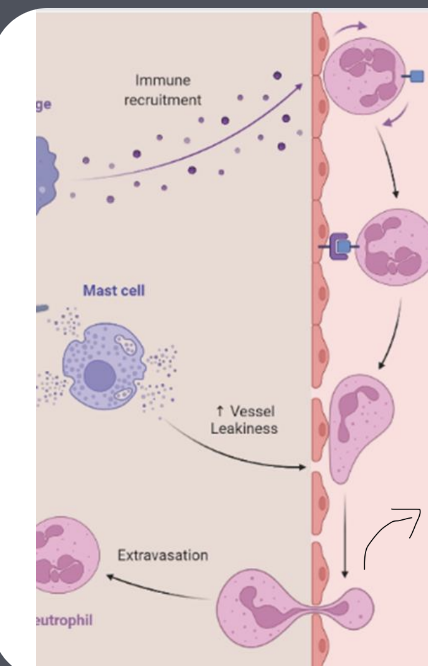
What are the tightly coordinated phases of immune cell infiltration?
Recruitment/capture - allow them to “grab onto the wall” of the blood vessel
Rolling
Crawling cells migrate along the blood vessel wall before entering tissues.
Transmigration (or diapedesis) - tension and stress allows for tiny gaps to be formed, that they can squeeze through
Name 2 vasoactive mediators that contribute to vasodilation and increased blood vessel permeability.
Histamine and Prostaglandins (PGs).
Where is histamine mainly found and from what amino acid is it synthesized?
It is a biogenic amine syntehsised from L-histidine. mainly found in mast cells.
How are PGs generated?
Generated from arachidonic acid by COX.
Which prostaglandin contributes to the main signs of inflammation (redness, swelling, and pain)?
PGE2, can be produced by a myriad of cells immune and non-immune (endothelial cells included)
What is chemotaxis?
Movement toward a chemical, or a gradient of accumulating chemical signals.
What are chemokines and when are they released?
Chemokines are chemotactic cytokines released during inflammation, injury, and infection that promote movement of immune cells
Engage chemokine receptors on the surface of immune cells
What two actions do chemokines promote when binding to receptors on innate immune cells?
Movement towards an area of high gradient
Increased binding affinity of integrins for their adhesion molecules.
Why is chemokine binding to receptors of innate immune cells a complex system?
Some receptors can bind more than one chemokine.
• Only if from corresponding type:
Some CCs can bind different CCRs and some CXCs various CXCRs.
How are chemokines produced?
• Can be produced by immune cells themselves, epithelium and stroma.
• Produced in response to PRR engagement or to cytokine stimulation
Which chemokines and receptors are key to attract neutrophils?
CXCL1-CXCR2
CXCL8-CXCR1 or CXCR2.
Which chemokine and receptor pair is key to attract monocytes?
CCL2-CCR2.
Which chemokine and receptor pair is key to attract dendritic cells?
CCL19-CCR7 or CCL21-CCR7.
What type of molecules are selectins, and what is their role in initial immune cell migration?
(initial adhesion molecules) They are carbohydrate-binding transmembrane proteins found in the surface of immune & endothelial cells that mediate relatively low affinity binding in the initial phase of migration.
Where is P-selectin (CD62P) distributed?
In the endothelium, activated by vasoactive mediators like histamine or thrombin produced by MC which act as stimuli for translocation of P-selectin to the cell surface, facilitating leukocyte adhesion.
Where is E-selectin (CD62E) distributed?
In the endothelium, induced by cytokines TNF, IL-1 during inflammation.
Where is L-selectin (CD62L) distributed?
Innate immune cells i.e: Neutrophils, monocytes, T cells (naïve + central memory), B cells
*Only found on leukocytes i.e surface of monocytes
Expression/confirmation of selectins is _________ in response to stimuli from ______ and __________
Increased, in response to cytokines and vasoactive peptides like histamine.
This promotes adhesion of leukocytes to the endothelium.
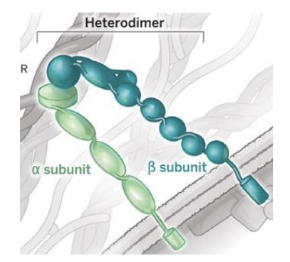
What are integrins and how do they facilitate immune cell infiltration ?
Integrins are proteins on immune cells surfaces which interact with adhesion molecules on endothelial and stromal cells,
This interaction allows anchoring of circulating immune cells to the blood vessel wall and allowing rolling and crawling.
Integrins are _____ formed by ___________
Dimers, formed by chains of alpha and beta subunits.
In the step-by-step process of immune cell infiltration, what is the sequence of events after vasoactive substances i.e histamine cause blood vessel dilation?
Blood flow slows, leukocytes move closer to vessel walls,
Selectin expression mediates initial binding
Cytokines/chemokines promote expression and conformational changes in adhesion molecules/integrins.
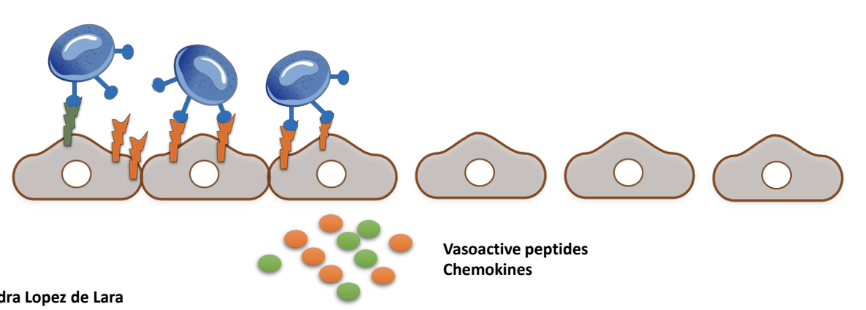
How do leukocytes enable transmigration through endothelial cells?
Leukocytes crawl on endothelial cells, & tether to the abundant adhesion molecule connections
These connections trigger processes inside endothelial cells that reduce paracellular connections
Facilitate their movement through the endothelial barrier, allowing them to exit the bloodstream and reach sites of inflammation.
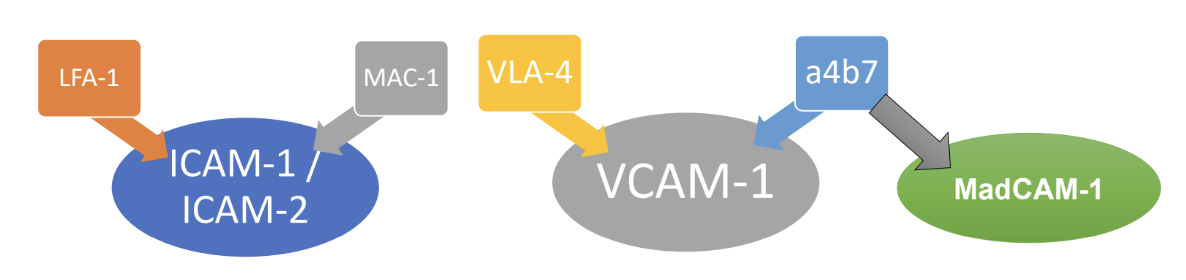
What processes do abundant adhesion molecule connections initiate in endothelial cells that reduce paracellular connections?
• Rearrangement of endothelial cell cytoskeleton, possibly favours their contraction.
• Phosphorylation of VE-cadherin and destabilization of cadherin-dependent junctions.
Triggered by abundant ICAM-1/VCAM-1-integrin connections.
What is meant by the paracellular route?
Immune cells move across endothelial walls using gaps created by the rearrangement of junctional proteins, allowing them to crawl between endothelial cells into tissues.
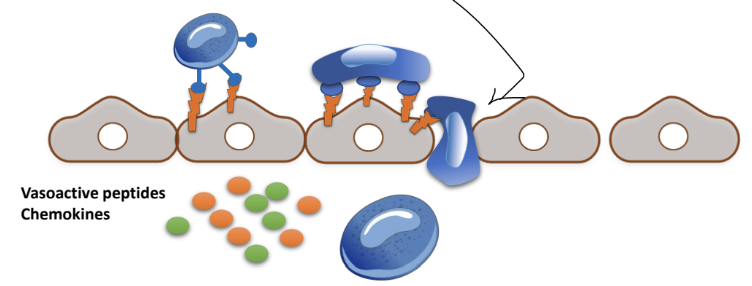
Leukocytes adhere and roll over the vascular wall: 1st contact via _____ , rolling via ______
selectins (weak adhesion), integrins (firm adhesion)
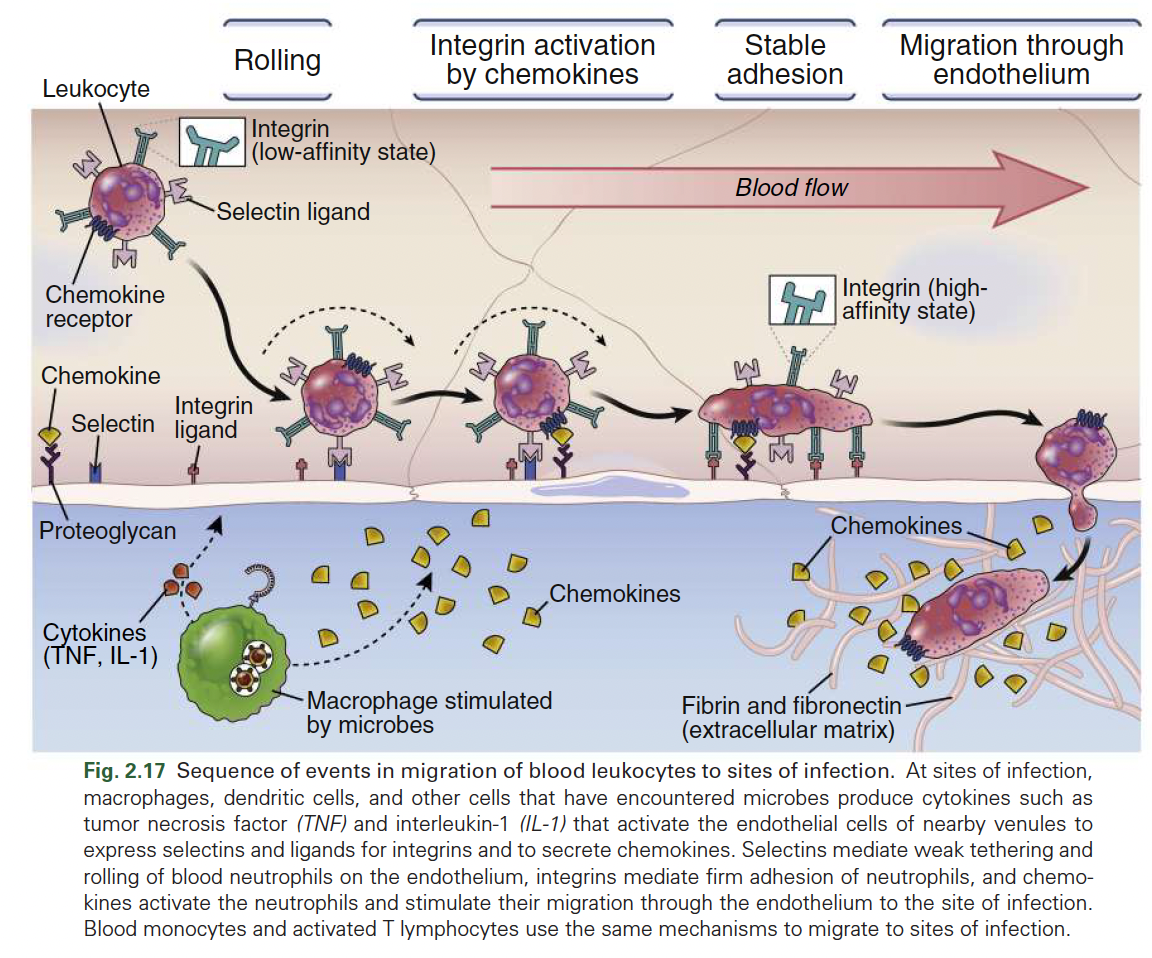
____________ binding relaxes connections between adjacent vascular endothelial cells. _______ is facilitated.
Histamine, prostaglandins and adherent, Oedema
Diapedesis
The process by which leukocytes squeeze between vascular endothelial cells to exit the bloodstream and enter tissues during inflammation.
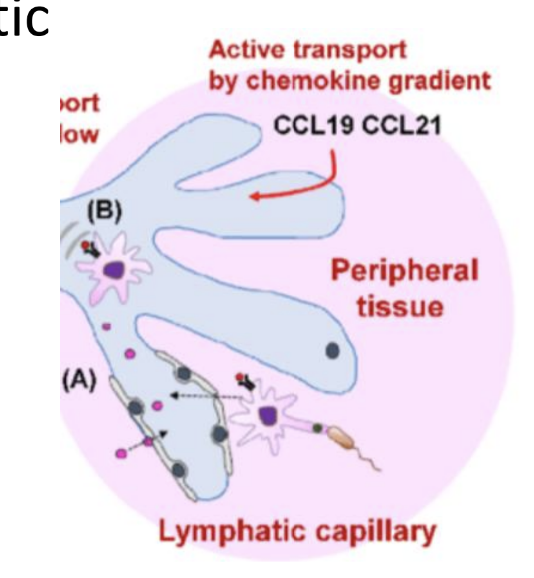
What leads to DC maturation?
Recognition of PAMPs and exposure to cytokines at sites of infection trigger phagocytosis and antigen processing of DC
What is the role of mature DC and what molecule coordinates this?
Migrate to nearby lymph nodes where they “educate” the adaptive immune system.
• Migration is coordinated by CCL21 released from lymphatic endothelium.
• DCs sense this via CCR7 receptor and migrate toward lymph nodes
What is inflammation?
A natural response of our organism against injury or infection that helps clear pathogens and promotes tissue repair.
Why is increasing blood flow in capillaries important during inflammation?
It enhances the delivery of immune cells, cytokines that help kill bacteria and nutrients to the site of injury, aiding in effective immune response and tissue healing.
How does chronic inflammation differ from physiologic inflammation?
Chronic inflammation is a deleterious, self-perpetuating response where excessive or sustained immune activation causes tissue destruction or abnormal repair,
unlike physiologic inflammation which leads to resolution and healing.
What is an autoimmune disease?
A condition where the immune system mistakenly attacks the body's own cells and host tissues, leading to tissue damage and a variety of symptoms.
What happens in Phase 1 (inflammation) of physiologic tissue repair after a barrier breach?
Barrier breach sensed by PAMPS / DAMPS
Resident immune cells release a cascade of chemokines, histamine, and PGs
This attracts circulating immune cells, causing vasodilation
Induces selectins/adhesion molecules and integrins, leading to infiltration of monocytes, granulocytes, and DCs.
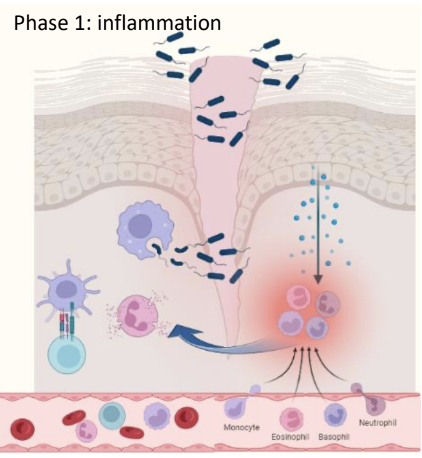
In Phase 1 of physiologic inflammation, what does the infiltration of monocytes do?
Release cytokines, differentiate to macrophages (Phagocytosis), ag presentation (Local).
In Phase 1 of physiologic inflammation, what does the infiltration of granulocytes do?
Degranulation: release of inflammatory mediators such as histamines and cytokines that help kill bacteria
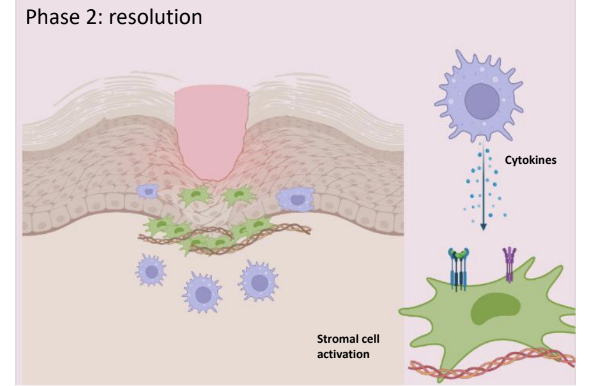
What occurs during Phase 2 (resolution) of physiologic tissue repair?
Infection is cleared
Granulocytes are destroyed
Monocytes/DCs may recirculate
Remaining MP stimulate healing
Stromal cells produce extracellular matrix i.e fibrin and epithelial cells regenerate to restore tissue architecture.
List some examples of diseases that result from inflammation getting out of control (pathologic inflammation).
Chronic inflammation, autoimmunity (e.g., IBD, Psoriasis, Rheumatoid arthritis, Multiple Sclerosis), transplant rejection, and allergies.
What are some factors that can trigger persistent activation of innate immune cells in chronic inflammation?
Genetic defects (e.g., NOD2 mutations in IBD)
Loss of tolerance toward commensal microbes
Defective epithelial barriers
Abnormal adaptive immunity (e.g., autoantibodies).
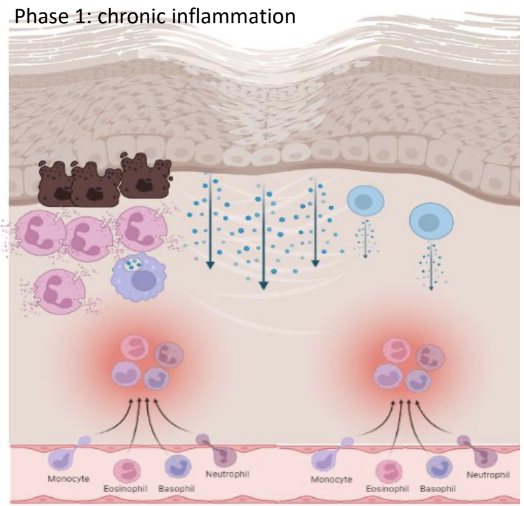
What occurs in phase 1 (chronic inflammation) in pathologic inflammation
Tissue destruction / Scarring:
• Continuous or enhanced recruitment of granulocytes: degranulation leads to epithelial damage, further breaches in the barrier.
• Accumulation of MP with enhanced production of cytokines, causing further activation of other immune and structural cells.
• MP products can also damage the epithelium.
• Adaptive immune cells are also recruited and activated, increasing cytokine production.
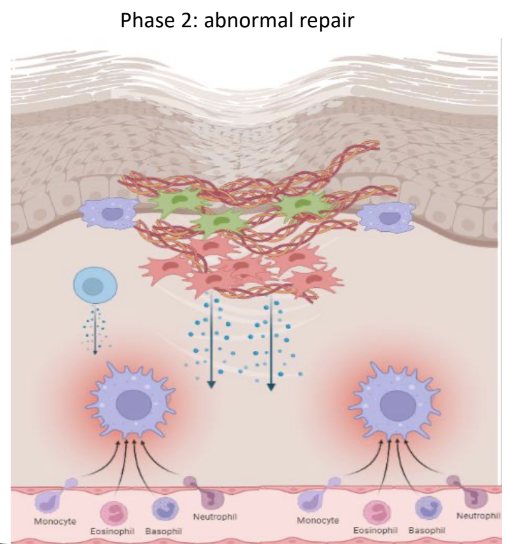
How does abnormal repair in pathologic inflammation lead to severe disease?
Overactivated local structural cells (like fibroblasts)
Lead to increased motility and tissue accumulation
Overproduction and deposition of ECM causing fibrosis and tissue strictures
Excessive epithelial damage causing ulcerations or fistulas.
Functions of prostaglandins + leukotrienes
Smooth muscle contraction
Increased vascular permeability
Promotion of chemotaxis of EP, BP and Th2 cells
Stimulates mucus secretion
Bronchoconstriction