Electrochemistry 3.1.11
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
47 Terms
How are equilibria set up in a solution of ions

What does electrode potential of the solution mean?

Diagram and equation showing what happens in a solution of metal ions
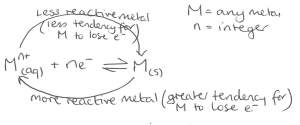
Examples of half equatios showing how equilibria determines metal positivity/negatitivy relative to solution
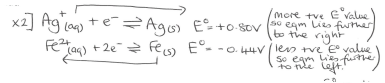
Overall equation for the examples from previous flashcard
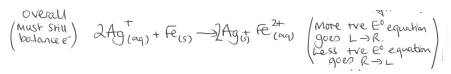
How is the potential difference between 2 metals found

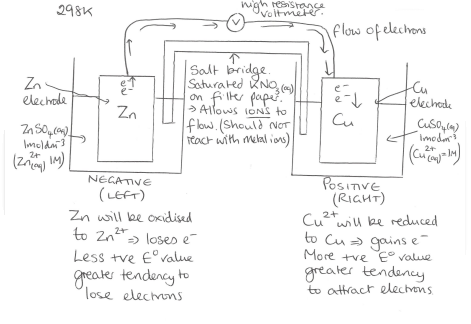
Diagram showing how an experiment is set up to show potential difference
How is the cell emf (potential difference) calculated

Example of the cell emf being calculated
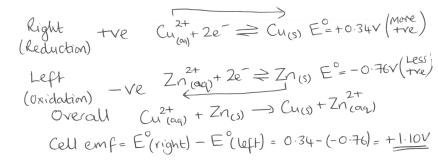
What is the standard electrode potential?

In previous example what would be the oxidising and reducing agents?
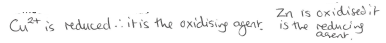
Diagram showing the conventional way a cell reaction is written/drawn
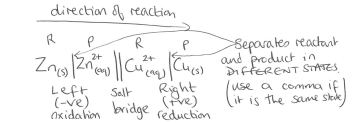
When is the reaction feasible for a cell reaction
How do you determine which ions in a electrode potential and oxidising/reducing agents
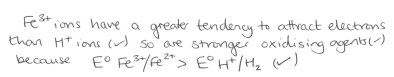
Why is hydrogen electrode used to measure the absolute electrode and what is the electrode potential assigned to

Diagram showing how the standard electrode potential can be found
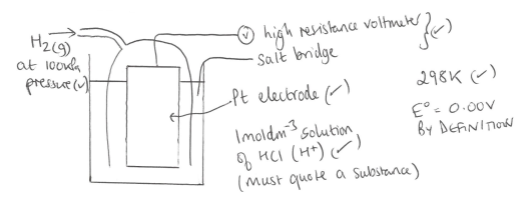
When investigating Eo values which electrode is the hydrogen electrode assigned to

What electrode could be used instead of the standard hydrogen electrode

Diagram for the electrochemistry series

How do Ion-ion systems work?
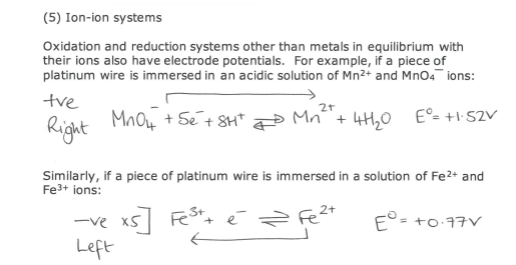
How to use Eo data to predict the direction of reaction

Equation linking e, n, f and delta G
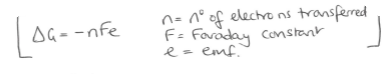
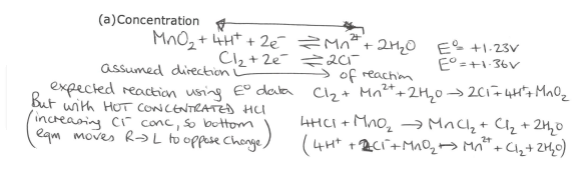
What happens when non standard conditions are used for predictions with Eo values? (concentration)
What happens when non standard conditions are used for predictions with Eo values? (Temperature)
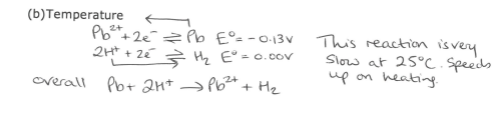
What happens when non standard conditions are used for predictions with Eo values? (Kinetic effects)
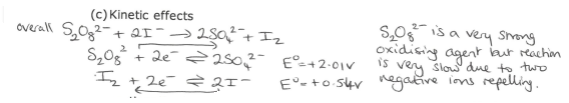
Why does the voltage of a non rechargable cell eventually drop?

Equations showing how a non rechargable (primary) zinc carbon cell works

Equations showing how a non rechargable alkali (primary) carbon cell works

What are the 2 key things about rechargable cells?

What are the equations for rechargable (secondary) cells -Li ion
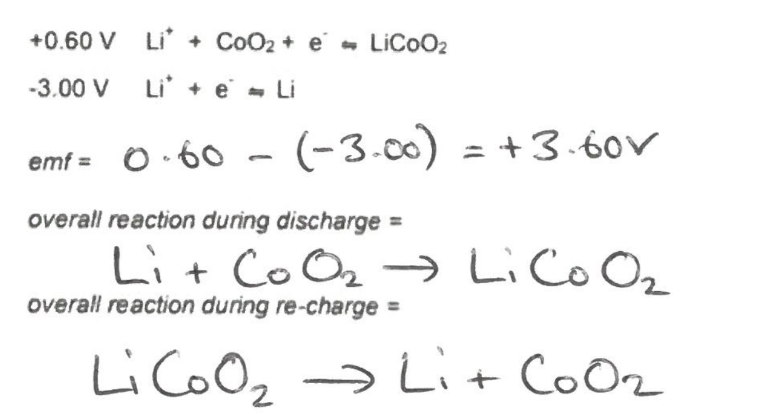
What are the equations for rechargable (secondary) cells - Lead acid
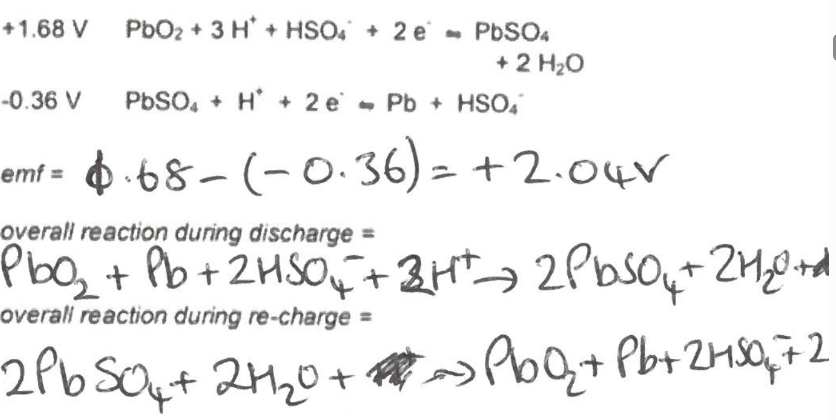
What are the equations of rechargable (secondary) cell nickle cadmium
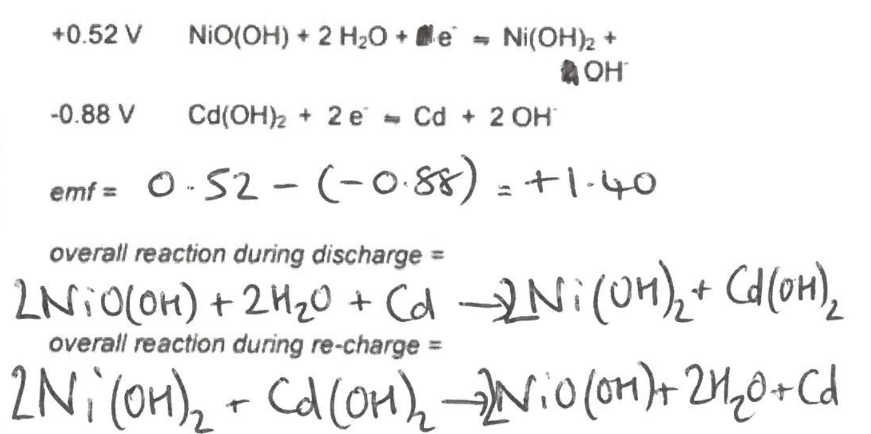
What is the key idea behind fuel cells

Diagram showing how Hydrogen and oxygen fuel cells work
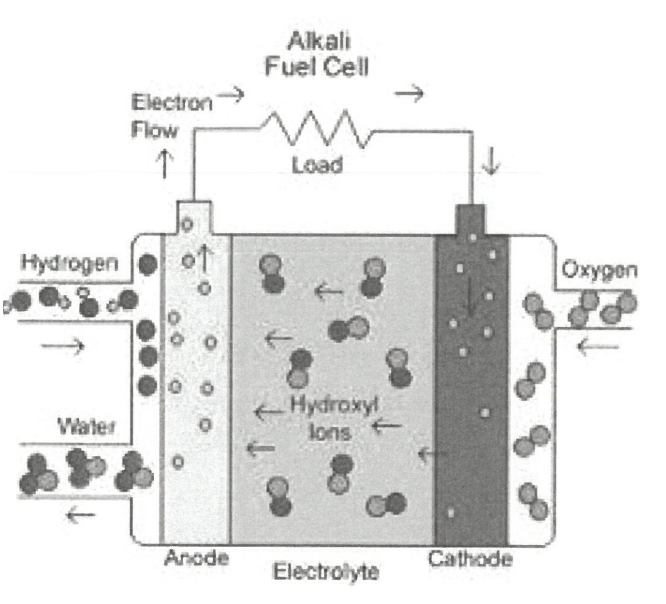
In a fuel cell where are the chemicals that generate electricity stored?

What is the difference between acidic and alkali fuel cells

how does a hydrogen oxygen fuel cell work and why is it beneficial that it works this way?

Where are the hydrogen and oxygen fed into the fuel cell and what are the equations for each electrode. What happens when electrons move within the fuel cell?

Pros and cons of using cells

Pros and cons of using non rechargable (primary) cells

Pros and cons of using rechargable (secondary) cells

Pros and cons of using fuel cells

What are half equations showing the reactions that happen at each electrode in a hydrogen , methane and octane fuel cell
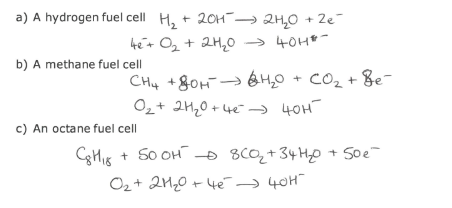
State 2 advantages of fuel cell vehicles over coventional vehicles

s
State 3 limitations of hydrogen fuel cells
State 3 reasons which may prevent a “hydrogen economy” from taking off
