cell-cell communication
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
89 Terms
types of cell signaling
paracrine, autocrine, and juxtracrine
paracrine
cells send out a signal to be received by competent cells
autocrine
variation where cell may respond to its own signal
juxtracrine
variation where cells must be in direct contact (hyperlocal)
endocrine signaling
hormone signaling
how does endocrine signaling work
travels through the blood
does endocrine signaling have a concentration gradient
no
does paracrine signaling have a concentration gradient
yes
induction
cells attempt to trigger a change in the responding cells
competence
not all cells may be able to respond to the signal; cells that can are competent
ligand
signal molecule who’s binding triggers a change in the receptor
ligand binding
activation typically involves conformational change, cleavage, and/or phosphorylation
transduction cascade
serial activation of intracellular signaling molecules
cell response
typically activation of a transcription factor and ensuring activation of new gene expression
cell signaling process
ligand binding activates the receptor (outside cell membrane)
activated receptor activates intracellular signaling molecules (between cell and nucleus membrane)
intracellular signaling activates effector - often a transcription factor (inside nuclear membrane)
activated transcription factor binds to DNA and activates transcription of a target gene
step one of cell signaling process
ligand binding activates the receptor (outside cell membrane)
step two of cell signaling process
activated receptor activates intracellular signaling molecules (between cell and nuclear membrane)
step three of cell signaling process
intracellular signaling activates effector - often a transcription factor (inside nuclear membrane)
step four of cell signaling process
activated transcription factor binds to DNA and activates transcription of a target gene
target gene
genes that have transcription activated when a signal pathway is activated
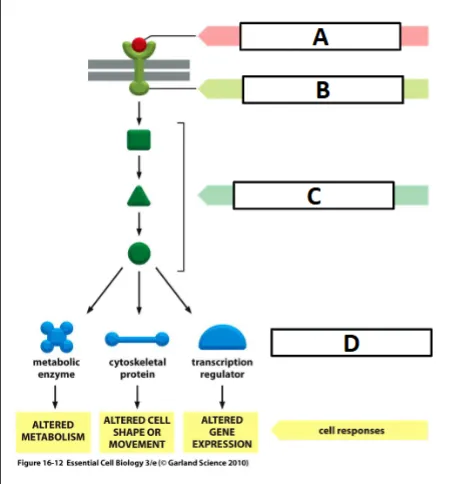
label the effector
D
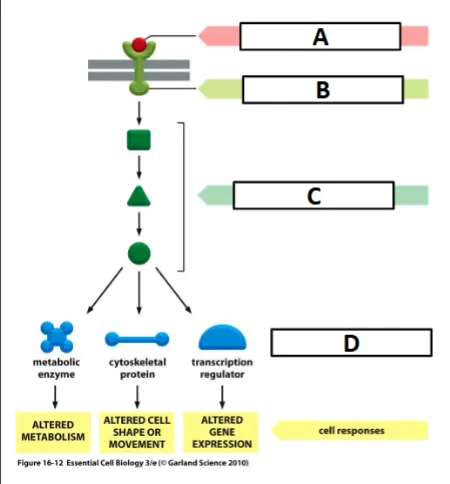
label the ligand
A
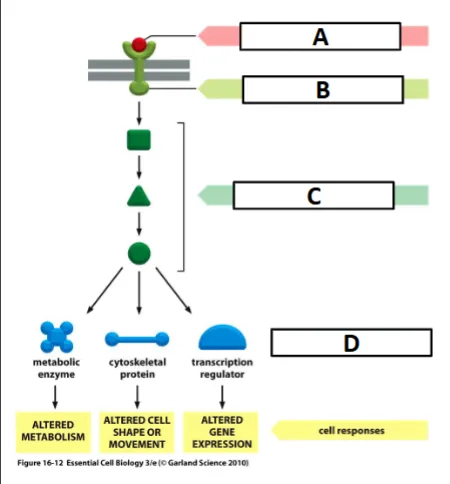
label the receptor
B
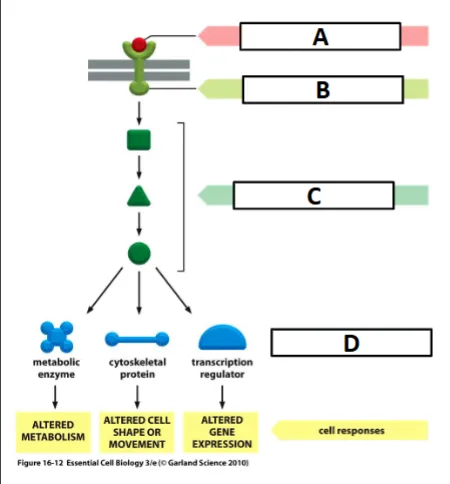
label the intracellular signaling molecules
C
key pathways
hedgehog, Wnt/wingless, TGF-β/BMP, FGF/RTK, and notch
hedgehog ligand
hedgehog (Hh), sonic hedgehog (Shh), indian hedgehog (Ihh)
hedgehog effector
transcription factor Ci (flies) or Gli (vertebrates)
what happens in the absence of hedgehog ligand
Ci/Gli is cleaved and cannot activate gene expression
where was the Hh pathway first discovered
Drosophila
what was Hh named after
mutant phenotype (embryo has extra bristles)
what is Hh role in development
important for roles in development of nervous system (spinal cord development and eye development) and limb development
what can impaired Hh signaling cause
cyclopia
is hedgehog paracrine, autocrine, or juxtracrine
paracrine
what is the hedgehog pathway target gene
Hh-target genes
Wnt ligand
Wnt (wingless/wg in flies)
Wnt effector
β-catenin transcription factor
what happens in absence of Wnt ligand
β-catenin is continually degradeed in the cytoplasm
what happens in the presence of Wnt
β-catenin is stabilized and can enter the nucleus to activate transcription of Wnt-target gene
what happens in the presence of the hedgehog ligand
Ci/Gli is not cleaved and can enter the nucleus, bind to enhancers, and activate expression of the Hh-target gene
what is the function of the Wnt pathway
important roles in early embryonic development (setting up early signaling centers like the Organizer) and kidney development
what do Wnt mutant mice lack
kidneys
is the Wnt pathway paracrine, autocrine, or juxtracrine
paracrine
what are the Wnt target genes
Wnt-target gene
TGF-β/BMP pathway ligand
TGF-β, BMPs, Nodal, Activin
TGF-β/BMP pathway internal signaling molecule
transcription factor called “Smads”
what does TGF-β/BMP ligand binding trigger
phosphorylation cascade to activate Smads
what happens in the absence of TGF-β/BMP ligands
Smads are not phosphorylated and are therefor inactive
what role does the TGF-β/BMP pathway play
important in early embryonic patterning (helps set up early patterning centers like the Organizer), limb development, and patterning of the nervous system
seen in BMP mutants
digit patterning defects (defect where digits don’t know which digit its meant to be)
is TGF-β/BMP pathway paracrine, autocrine, or juxtracrine
paracrine
FGF/RTK pathway ligands
growth factors (GFs) such as FGF, EGF, VEGF, etc.
FGF/RTK pathway receptors
growth factor receptors (GFRs) such as FGFR, EGFR, VEGFR, etc.
what internal signaling molecules are often mutated in human cancer
Ras and Raf
what does FGF/RTK ligand binding trigger
phosphorylation of internal signaling molecules
what pathways trigger phosphorylation
FGF/RTK pathway and TGF-β/BMP pathway
FGF/RTK effector proteins
transcription factors
FGF/RTK target genes
pro-proliferation genes
FGF/RTK role
important in kidney development, vasculogenesis, and craniofacial development
what does impaired vasculogenesis in VEGF mutant look like
swelling of the limbs
is the FGF/RTK pathway paracrine, autocrine, or juxtracrine
paracrine
is the notch pathway paracrine, autocrine, or juxtracrine
juxtracrine
notch pathway ligands
delta, jagged
what type of protein are notch pathway ligands
membrane proteins
notch pathway receptor
notch
what happens in the absence of the notch pathway ligand
the notch protein is embedded in the membrane
what happens in when the notch pathway ligand binds
the interior portion of the notch protein is cleaved and is free to travel to the nucleus, where it acts as a transcription factor
what is the role of the notch pathway
important for somite/vertebral development (backbone) and hematopoiesis
what can a mutation in the notch pathway lead to
vertebral defects such as “butterfly” vertebrae
when was the Townes and Holtfreter experiment
1995
what was the Townes and Holtfreter experiment
took 2 different frog species’ embryos and spliced them together (epithelial from one and neural from another), mixed them together and the cells eventually self sorted to a neural center with an epithelial shell
Which of the pathways discussed today (Hh, Wnt/Wg, TGF-β/BMP, FGF/RTK, and Notch) is NOT a paracrine signaling pathway
notch - it is juxtracrine
What is the transcription factor in the Hh pathway
Ci/Gli
What is the transcription factor in the Wnt/Wg pathway
β-catenin
“Smads” are intracellular signaling molecules in which pathway
TGF-β/BMP
which pathway is often mutated in human cancers
FGF/RTK - bc of its association with growth factors
cadherins
proteins on the surface of cells that mediate cell-cell attraction/repulsion
what type of cadherins attract
same type
what type of cadherins repel
different types
cadherins produced by epithelial cells
E-cadherin
cadherins produced by nervous tissue
N-cadherins
what regulates strength of addhision
cadherin concentration
stronger cadherin concentration
more central tight location in embryo
weaker cadherin concentration
outer layer of embryo
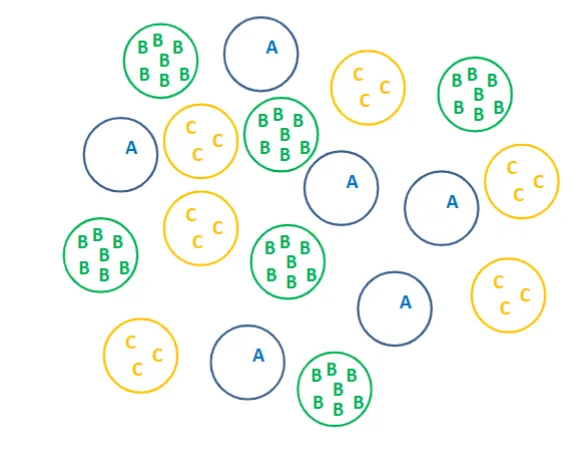
how would these cells organize after being mixed together
B-cells would be in the center, C-cells would be the middle layer, and A-cells would be the outer layer
what can changes in cadherin expression drive
morphological events
how does cadherin expression change as a cell separates from one tissues and migrates to another
the cadherin expression gets weaker
epithelial-to-mesenchymal transition (EMT)
when cells that are originally attached to one another (as part of the epithelium) separate and migrate away
what happens when a tumor becomes metastatic
part of the tumor has undergone an inappropriate EMT
what cancers is the downregulation of cadherin expression linked to
breast, lung, and oral cancer