Complete set
0.0(0)
0.0(0)
Card Sorting
1/174
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
175 Terms
1
New cards
hypothesis
proposed explanation made from obseravation
2
New cards
theory
set of tested hypotheses that provides general explanation of a natural phenomenon
3
New cards
scientific law
observed behavior that had no exceptions
4
New cards
mass
quantity that represents the amount of matter a object or substance has
5
New cards
matter
substance that makes up a physical object
6
New cards
element
substance that cannot be broken down into other substances
7
New cards
compound
substance formed from two or more elements chemically bonded
8
New cards
homogenous mixture
mixture with a uniform composition and no visibly distinguishable parts
9
New cards
heterogeneous mixture
mixture without a uniform composition, visibly distinguishable parts
10
New cards
physical change
change in the appearance or form of the matter of a substance
11
New cards
chemical change
change of one substance into another with different properties
12
New cards
atom
basic unit of a chemical element that can enter a chemical combination
13
New cards
molecule
a group of atoms bonded together; smallest fundamental unit of a chemical compound
14
New cards
kilo
1000
15
New cards
pico
0\.0000000000001
16
New cards
mili
0\.001
17
New cards
centi
0\.01
18
New cards
mega
1,000,000
19
New cards
nano
0\.0000000001
20
New cards
zeros to the left of non-zero digits
not significant; tells magnitude
21
New cards
zeros between non-zero digits
always significant
22
New cards
zeros to the right of non-zero digits
significant if to the right of the decimal
23
New cards
density
degree of compactness of a substance
24
New cards
25
New cards
ion
anything that has a charge to it
26
New cards
cation
positively charge metal ions
27
New cards
anion
negative charge ions
28
New cards
nucleus
center of atom (contains protons and neutrons)
29
New cards
atomic number
the number of protons in an element
30
New cards
mass number
the weight of an atom in amu
31
New cards
isotopes
varying versions of an element with different numbers of neutrons that naturally occur
32
New cards
electronegativity
ability of an atom in a covalent bond to attract electrons
33
New cards
reactant
substances that start a chemical reaction
34
New cards
product
what is produced in a reaction
35
New cards
oxidation number
total number of electrons an atom gains or loses when forming chemical bonds
36
New cards
oxidation
process or results of an element combining with oxygen; will lose electrons
37
New cards
reduction
process or result of being reduced or gaining electrons
38
New cards
acid
compound that increase H+ ion concentration in an aqueous solution
39
New cards
base
compound that increase OH- ion concentration in an aqueous solutions
40
New cards
salt
ionically bonded compound
41
New cards
neutralization
chemical reaction when an acid and base react with each other
42
New cards
precipitation reaction
two aqueous solutions react to form one or more solid products
43
New cards
combustion
reaction with oxygen to produce heat or light
44
New cards
decomposition reaction
reaction where a single reactant breaks down to two or more simpler compounds
45
New cards
lithium is a…
active metal
46
New cards
sodium is a…
active metal
47
New cards
potassium is a…
active metal
48
New cards
cesium is a…
active metal
49
New cards
rubidium is a…
active metal
50
New cards
calcium is a…
active metal
51
New cards
strontium is a…
active metal
52
New cards
barium is a…
active metal
53
New cards
lithium hydroxide acts as a…
base
54
New cards
sodium hydroxide acts as a…
base
55
New cards
potassium hydroxide acts as a…
base
56
New cards
calcium hydroxide acts as a…
base
57
New cards
barium hydroxide acts as a…
base
58
New cards
strontium hydroxide acts as a…
base
59
New cards
hydrogen fluoride is a nonmetal H compound that acts as an…
acid
60
New cards
hydrogen chloride is a nonmetal H compound that acts as an…
acid
61
New cards
hydrogen bromide is a nonmetal H compound that acts as an…
acid
62
New cards
hydrogen iodide is a nonmetal H compound that acts as an…
acid
63
New cards

\
hydrochloric acid
64
New cards

sulfuric acid
65
New cards
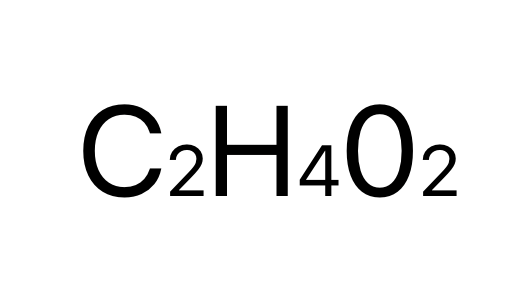
acetic acid
66
New cards
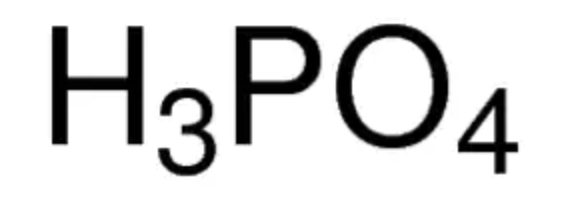
phosphoric acid
67
New cards
hydrochloric acid with barium hydroxide is a…
neutralization reaction
68
New cards
potassium and water is a…
redox reaction
69
New cards
barium chloride (aq) and sulfate (aq) is a…
precipitation reaction
70
New cards
metals can conduct heat and electricity …
easily
71
New cards
at room temp. metals are…
solid
72
New cards
when freshly cut metals are…
shiny
73
New cards
positively charged ion formed by metals
cation
74
New cards
nonmetal do not..
conduct electricity or heat
75
New cards
metals can be deformed without breaking making them….
adaptable
76
New cards
nonmetals are not adaptable so they can’t…
be deformed without breaking
77
New cards
at room temp. nonmetals are
gas or liquid
78
New cards
negatively charged ions made by nonmetals
anion
79
New cards
alkali metals are in group…
1A
80
New cards
alkaline earth metals are in group…
2A
81
New cards
halogens are in group…
7A
82
New cards
noble gases are in group…
8A
83
New cards
lanthanides are in period…
6
84
New cards
actinides are in period…
7
85
New cards
transition metals are in groups…
3-12
86
New cards
energy
ability to do work or produce heat
87
New cards
work
force acting over some distance
88
New cards
heat
transfer of energy between two object due to temperature differences
89
New cards
1 cal \=
4.184 J
90
New cards
temperature
measure of kinetic energy an object has
91
New cards
system
what you are observing (reaction vessel)
92
New cards
surroundings
everything that isn’t being observed
93
New cards
state function
independent of the pathway taken
94
New cards
Delta Y
indicates a state function
95
New cards
enthalpy is a…
state function
96
New cards
1st Law of Thermodynamics
energy cannot be created or destroyed only transferred
97
New cards
Delta E
sum of all internal energy
98
New cards
Equation for enthalpy change
delta H \= E + PV
99
New cards
Delta H is equal to Delta E when…
there are no gaseous reactants or products
100
New cards
exothermic
energy is released