ELECTRONIC STRUCTURE & PERIODIC PROPERTIES OF ELEMENTS
0.0(0)
0.0(0)
Card Sorting
1/124
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
125 Terms
1
New cards
1A
alkali metals
2
New cards
2A
alkaline earth metals
3
New cards
B
transition metals
4
New cards
5A
Pnictogens
5
New cards
6A
Chalcogens
6
New cards
7A
Halogens
7
New cards
8A
Noble gases
8
New cards
Metals
good conductors of heat and electricity \n malleable \n ductile \n shiny \n most are solid \n except murcury
9
New cards
Nonmetals
poor conductors of heat and electricity
many colors \n solids, liquids, gases \n better insulators
many colors \n solids, liquids, gases \n better insulators
10
New cards
Wavelength
The distance between two corresponding parts of a wave
11
New cards
Frequency
(v) how often a new wave arrives at a point
12
New cards
Amplitude
(A) height of wave from midline to peak or trough
13
New cards
relationship of wavelength and frequency
inversely proportional as one goes up the other goes down
c= (landa)\* V
c= (landa)\* V
14
New cards
Constructive interference
The interference that occurs when two waves combine to make a wave with a larger amplitude
15
New cards
Destructive interference
The interference that occurs when two waves combine to make a wave with a smaller amplitude
16
New cards
Quantization
only those waves having an integer, n, of half-wavelengths between the end points can form
17
New cards
Nodes
one or more points between the two end points that are not in motion
where amplitude=0
nodes-1
where amplitude=0
nodes-1
18
New cards
radial nodes
all angles, constant radii (circles)
19
New cards
angular nodes
all radii, constant angles (lines)
20
New cards
Blackbody radiation
higher temperatures lead to greater intensity and peak shifting to shorter wavelengths
\
but there is a steep drop-off in the ultraviolet region
\
The peaks are in infrared at lower temps and peaks at ultraviolet/visible at increase temp
\
but there is a steep drop-off in the ultraviolet region
\
The peaks are in infrared at lower temps and peaks at ultraviolet/visible at increase temp
21
New cards
The photoelectric effect
light that meets certain energy/frequency requirements is able to eject electrons from a metal surface
\
indicates that light can act like particles called photons
\
the energy of photons depends on frequency
\
E=hv
\
indicates that light can act like particles called photons
\
the energy of photons depends on frequency
\
E=hv
22
New cards
Line Spectra
the light emission only at specific wavelengths (because its quantized)
\
Wavelength can be calculated for each of the lines in the line spectrum using the Rydberg equation
\
Wavelength can be calculated for each of the lines in the line spectrum using the Rydberg equation
23
New cards
The Bohr Model
explains the line spectrum of the hydrogen atom
\
In his theory of the atom, electrons are found in "orbits" around the nucleus
\
Orbits that are farther out are higher in energy
\
In his theory of the atom, electrons are found in "orbits" around the nucleus
\
Orbits that are farther out are higher in energy
24
New cards
Absorption
an electron moves to higher energy as light is positive
25
New cards
Emission
electrons moves to lower energy as light is negative
26
New cards
Ground state
The lowest energy state of an atom
27
New cards
Excited state
A state in which an atom has a higher potential energy than it has in its ground state
28
New cards
Rydberg Formula
\
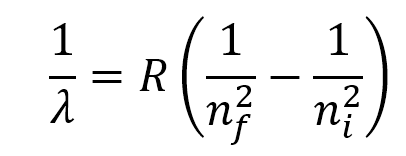
29
New cards
Development of quantum theory
particles and waves behave very differently on a macroscopic scale
\
there is no clear separation between particles and waves
\
there is no clear separation between particles and waves
30
New cards
DeBroglie wavelength
λ\=h/mv
31
New cards
DeBroglie Mass
m\=h/λv
32
New cards
Heisenberg Uncertainty Principle:
we can't know exactly where an electron is, but we can predict regions where it is most likely to be found
33
New cards
Electron interference patterns
wave-particle duality seen with electron interference patterns
\
with a few electrons, you can see the individuality of the particles
\
with many electrons, you can see the wave-like patterns
\
with a few electrons, you can see the individuality of the particles
\
with many electrons, you can see the wave-like patterns
34
New cards
Orbital
the region where an electron is likely to be found
35
New cards
Schrodinger equation
mathematically describes the orbitals in a hydrogen atom
36
New cards
Quantum numbers overview
tells us the size, shape, and orientation of the orbital each electron is in
37
New cards
Principal quantum \#
n, size/energy level
\
corresponds to the n # in the Bohr's model
\
corresponds to the n # in the Bohr's model
38
New cards
angular momentum quantum number
symbolized by l, indicates the shape of the orbital
\
0-s, 1-p, 2-d, 3-f
\
0-s, 1-p, 2-d, 3-f
39
New cards
magnetic quantum number
symbolized by m, indicates the orientation of an orbital around the nucleus
40
New cards
Electron spin quantum number
(ms)
\
Can be +1/2 (spin up) or -1/2 (spin down)
\
Related to whether electron is spinning clockwise or counterclockwise
\
Need n, l, ml , and ms to describe a particular electron within an orbital
\
There can be up to 2 electrons per orbital
\
Can be +1/2 (spin up) or -1/2 (spin down)
\
Related to whether electron is spinning clockwise or counterclockwise
\
Need n, l, ml , and ms to describe a particular electron within an orbital
\
There can be up to 2 electrons per orbital
41
New cards
How to find the \# of radial nodes
n-L-1
42
New cards
S
L- 0
ml- -1,0,1 \n orbitals- s-1 \n spheres
ml- -1,0,1 \n orbitals- s-1 \n spheres
43
New cards
P
L- 1 \n ml- -1,0,1 \n orbitals- px,py,pz -3 orbitals \n dumbbells
44
New cards
d
L- 2 \n ml- -2,-1,0,1,2 \n orbitals- dxy, dxz, dyz, dz2, dx2,- 5 orbitals \n clovers
45
New cards
f
L- 3 \n ml- -3,-2,-1,0,1,2,3 \n orbitals- 7 different f orbitals
46
New cards
Pauli Exclusion Principle
no two electrons can have the same exact set of quantum numbers
\
each set of 4 quantum numbers describes one electron just like each address describes how to find one person
\
each set of 4 quantum numbers describes one electron just like each address describes how to find one person
47
New cards
Aufbau Principle
Fill in electron configurations and orbital diagrams starting with lowest energy orbitals
\
whenever you have a choice between a lower energy orbital and a higher energy orbital, choose the lower energy one
\
whenever you have a choice between a lower energy orbital and a higher energy orbital, choose the lower energy one
48
New cards
Hund's rule
for orbitals of the same energy, maximize the number of unpaired electrons
49
New cards
Valence
outer shell (highest n) electrons
50
New cards
Core
inner (lower n) electrons
51
New cards
Exceptions:
Chromium to make half-full d subshell (4s1 3d5) and Copper to make full d subshell (4s1 3d10)
52
New cards
Electron configuration with anion
add electrons using filling rules
53
New cards
electron configuration with cation
take away electrons from the highest n first
54
New cards
Isoelectronic atoms and ion
when they have the same number of electrons and electron configuration
55
New cards
effective nuclear charge (Zeff)
amount of positive charge from the nucleus that an electron "feels"
\
z (atomic number) - shielding (core electrons do the shielding)
\
Core electrons shield better than outer electrons
\
Increases across the period because core electrons remain the same but Z increases
\
z (atomic number) - shielding (core electrons do the shielding)
\
Core electrons shield better than outer electrons
\
Increases across the period because core electrons remain the same but Z increases
56
New cards
Trend in radius
larger when moving down and left
The less protons and more electrons sticking out will increase radius \n \n The more protons than electrons the more the electrons will be pulled in by protons and radius will decrease \n \n As # of energy levels increases, electrons are farther from the nucleus and feel less attractive forces (less pull inwards by protons)
The less protons and more electrons sticking out will increase radius \n \n The more protons than electrons the more the electrons will be pulled in by protons and radius will decrease \n \n As # of energy levels increases, electrons are farther from the nucleus and feel less attractive forces (less pull inwards by protons)
57
New cards
Groups
columns on the periodic table
58
New cards
Periods
rows on the periodic table
59
New cards
Ionic radius anion
\-Adding electrons makes radius bigger (more electron-electron repulsions) anion
\
larger than neutral bc of electron-electron repulsions
\
larger than neutral bc of electron-electron repulsions
60
New cards
Ionic radius cation
\-Removing electrons makes radius smaller (easier for protons to pull electrons inwards) cation
\-Removing all valence electrons makes it MUCH smaller (going from larger n to smaller n) \n - smaller than neutral
\-Removing all valence electrons makes it MUCH smaller (going from larger n to smaller n) \n - smaller than neutral
61
New cards
Ionic radius trend
increases as you go down and left
\
\-For isoelectronic series (different # of protons but same # of electrons), radius decreases with increasing Z
\
\-For isoelectronic series (different # of protons but same # of electrons), radius decreases with increasing Z
62
New cards
Ionization energy
The amount of energy required to remove an electron from an atom in the gas phase
\
the result is a cation with a positive charge
\
upper right= higher number = harder to remove
\
lower left=lower number=easier to remove
\
the result is a cation with a positive charge
\
upper right= higher number = harder to remove
\
lower left=lower number=easier to remove
63
New cards
Exceptions of ionization energy trend (3A)
Ionization energy slightly decreases from 2A to 3A
64
New cards
Exceptions of ionization energy trend (6A)
ionization slightly decreases from 5A to 6A because it is easier to remove a paired electron from the 6A
65
New cards
Why does it take more energy to remove core electrons than valence electrons?
because they feel more attraction to the nucleus and there is less shielding
66
New cards
Which electron is the hardest to remove when having 4 valence electrons?
the 4th IE4
67
New cards
Electron Affinity
the energy change for the process of adding an electron to a neutral atom in the gaseous state to form a negative ion (anion)
68
New cards
Electron affinity trend
increases as it goes to upper right but the exception is the noble gases because they do not want electrons
69
New cards
Ionic compounds
3D array of ions, made of cations and anions held together by electrostatically
\
they can make single elements or multiple elements
\
they can make single elements or multiple elements
70
New cards
Molecular compounds
covalent bonded compounds
71
New cards
Monoatomic
single element ion compound
72
New cards
Polyatomic
multiple atoms of ionic compounds
73
New cards
What are metals most likely to become and why?
cations because they tend to lose electrons and they have low ionization energy so that they can have the same number of electrons as a noble gas
74
New cards
What are non metals most likely to become and why?
anions because they have high electron affinities
\
This is so they have the same number of electrons as a nobel gas
\
This is so they have the same number of electrons as a nobel gas
75
New cards
Cations charge
charge \= group \#
76
New cards
anions charge
charge\=group \#-8
77
New cards
Perchlorate
ClO4-
78
New cards
Chlorate
ClO3-
79
New cards
Chlorite
ClO2-
80
New cards
Hypochlorite
ClO-
81
New cards
nitrate
NO3-
82
New cards
nitrite
NO2-
83
New cards
sulfate
SO4 2-
84
New cards
sulfite
SO3 2-
85
New cards
phosphate
PO4 3-
86
New cards
phosphite
PO3 3-
87
New cards
perchloric acid
HClO4
88
New cards
Chloric acid
HClO3
89
New cards
chlorous acid
HClO2
90
New cards
hypochlorous acid
HClO
91
New cards
nitric acid
HNO3
92
New cards
nitrous acid
HNO2
93
New cards
sulfuric acid
H2SO4
94
New cards
sulfurous acid
H2SO3
95
New cards
phosphoric acid
H3PO4
96
New cards
Phosphorous acid
H3PO3
97
New cards
Hydronium
H3O+
98
New cards
water
H2O
99
New cards
hydroxide
OH-
100
New cards
Sulfate
SO4 2-