CH164
1/145
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
146 Terms
electronegativity definition
a measure of the tendency of an atom to attract a bonding pair of electrons
oxidation state model
electrons are fully passed to one molecule
just a formality
covalent bonding
atoms are held together by a strong electrostatic attraction between the shared pair of electrons and the nuclei of the bonded atoms
extreme model
factors that increase bond strength of covalent bonding
short bonds
large difference in electronegativity between the atoms
large degree of polarisation
strong ionic contribution to bonding
how strong is a hydrogen bond
average bond enthalpy = 5-30 kJ/mol
in water H-bonding is 22x weaker than covalent bond in O-H
dative bonds
atom which donates both electrons becomes positively charged
ionic bonding
not directional - just need to be close
good description when Δχ is large
allows assignment of oxidation states
ionic lattice strength
600 - 4000 kJ/mol
high charges and small ions = strongest lattice enthalpy
metallic bonding melting points
heavier metals = higher melting points
what’s included in intermolecular interactions
combination of permanent dipole-permanent dipole, permanent dipole-induced dipole, temporary dipole-induced dipole
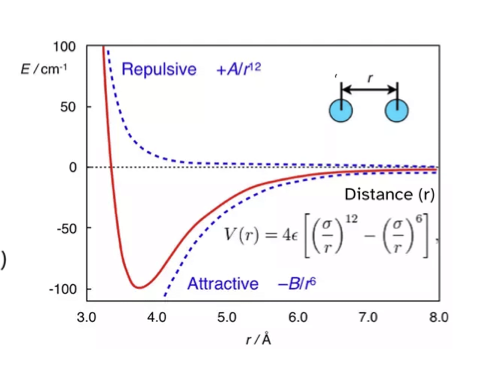
London dispersion forces
rate of decrease
factors to increase strength
ratio of London forces to other forces
temporary dipole - induced dipole interactions
decrease at rate of 1/r6 (r = distance between atoms)
heavier elements have larger clouds of electron density so are more polarisable, increasing boiling point
responsible for 100% of interactions between identical non-polar molecules but roughly 35% if it is a polar molecule
Lennard-jones potential
V( r ) = A/r12 - B/r6
what are intramolecular interactions composed of and their strengths
electrostatic interactions between charges
proportional to 1/r
bigger charge = stronger
dipole-dipole interactions
proportional to 1/r3
bigger dipole = stronger
dispersion interactions
proportional to 1/r6
bigger atoms = stronger
steric repulsion at very short range
proportional to 1/r12
clashes between electrons
examples of exotic bonding
halogen bonding
metallophilic interactions
chalcogen bonding
quadrupole interaction (pi-pi stacking)
what are robust bonding models able to do
explain systems
predict observations
be consistent across contexts
be consistent with other scientific models used
lewis theory/valence bond theory
assumes electrons in a bond are localised between two nuclei
dot & cross diagrams rules
all electrons in a molecule want to form a pair
a leftover single electron indicates the species is a radical
aim for octet
negatives of dot & cross diagrams
drawing double bond suggests that it is 2x single bond when it isn’t
doesn’t provide shape of a molecule
all bonds are the same
octet rule
an atom forms bonds in order to lose, gain or share electrons to give an outer shell containing 8 electrons, to achieve noble gas configuration
useful for s & p block atoms
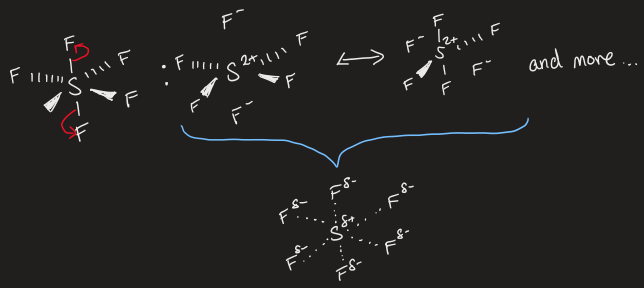
heavier elements appear to expand the octet or become hypervalent
lewis structure rules
make up octets
draw one covalent bond between connecting atoms
check formal charge on each atom
dative bonds make structures formally charged
not always entirely accurate but a tool for describing structures
lewis acid/base
lewis acid has an empty orbital
lewis base has a lone pair
bond order
number of bonding electrons/2
can be decimals or fractions
molecular orbital theory
electrons exist in molecular orbitals spread across the molecule
2 electrons per molecular orbital
bonding and anti-bonding possible
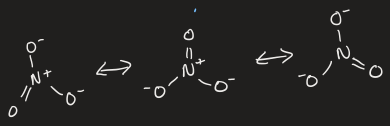
resonance
describes delocalised electrons within a molecule, when one single lewis structure cannot express the bonding
molecule is an average of the resonance forms
electroneutrality principle
each atom in a stable substance has a charge close to 0
draw resonance structures of [NO3]-
resonance of SF6 (expanded octet)
bond order < 1
in this case bond order = 2/3
order of magnitude for different repulsions for VSEPR
lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair
triple bond > double or single bonds
what causes decrease in repulsion
greater difference in electronegativity
longer bonds
electron density is pulled away from central atom through bonding
negatives of VSEPR
doesn’t account for differing sizes of atoms (steric factors)
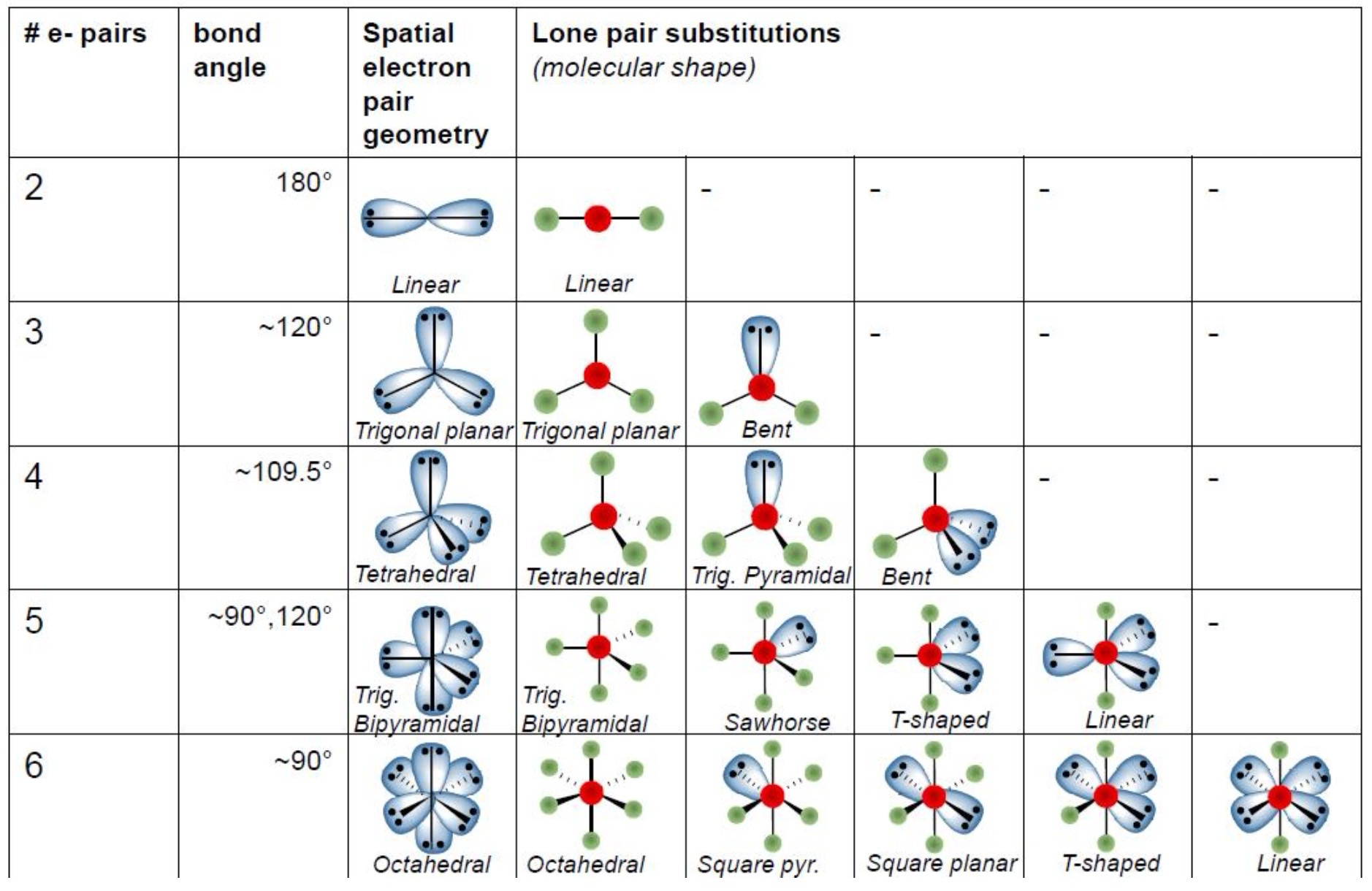
steps for predicting shapes using VSEPR
draw lewis structures
identify central atoms
identify number of valence electron pairs on central atom
work out parent geometry
place lone pairs in least crowded sites
place bonds elsewhere
assign a name to the shape of the molecule
names:

limitations of VSEPR/lewis theory
some structures have similar energies
doesn’t predicted magnetic properties
e.g. O2 is paramagnetic but VSEPR suggests its diamagnetic
doesn’t work well for when valence pairs = 7
doesn’t work for d-block compounds
doesn’t account for steric factors
symmetry
the quality of being made up of exactly similar parts faving each other or around an axis
a symmetry operator
an action that leaves the object apparently indistinguishable
a proper symmetry operation
can be done in real like (only rotational) to a physical shape
a symmetry element
the line or plane about which the symmetry operator is performed
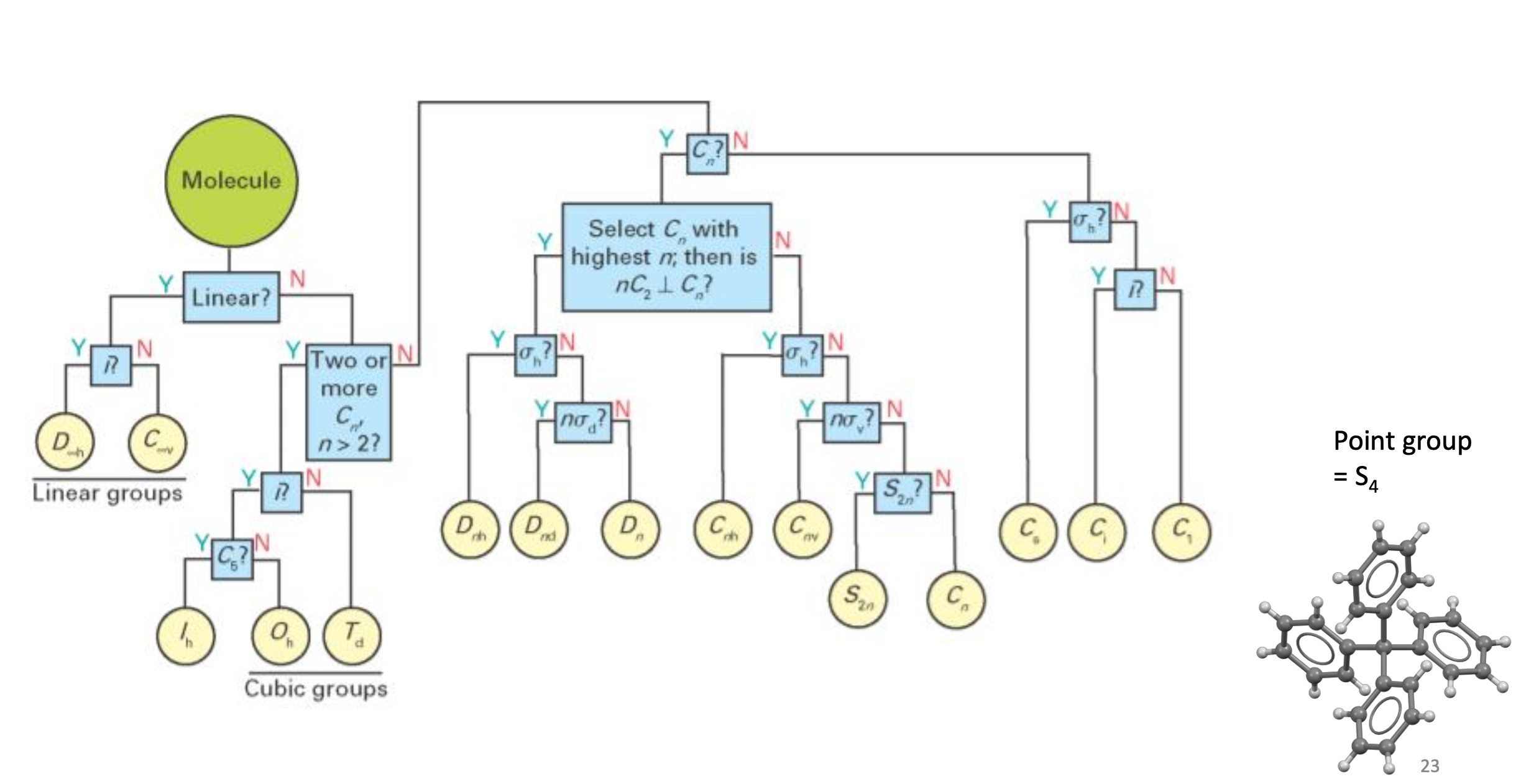
what is Cn and what do the letters stand for
C = rotation
n = how many times you rotate it
largest n = principle axis (vertical)
what is C∞
can rotate it infinite amount
for all linear molecules
what is σ (point groups)
reflectional symmetry
σh = reflection horizontal to principle axis
σd = reflection dihedral between the corners (between atoms)
σv = reflection vertical to the principle axis (through atoms)
what is Sn
improper rotation
Cn followed by σ reflection
same n as Cn
what is .i
inversion
S2 improper rotation
turns molecule inside out
point group
when two molecules possess the same set of symmetry elements
how to label a point group
Number = order of principle axis
Capital letter
D = n C2 axes at right angles to principle axis
E.g. for benzene there are 6xC2 which are horizontal to the principle axis so it is D
C = no n C2 axes at right angles to principle Cn axis
Small letter
h = horizontal mirror plane
d, v = no h but n vertical mirror planes
d goes with D
v goes with C

polar molecule
has a dipole moment caused by non-symmetric polar bonds
a dipole moment
a property of the molecule
unchanged by symmetry operation
cannot be perpendicular to any Cn axis
cannot occur if it has a centre of inversion
chiral compounds
non-superimposable on their mirror image
rotates the plane of polarised light
does not possess axis of improper rotation
what are symmetry adapted linear combinations (SALCs) used for
important in molecular orbital theory
calculated for the orbitals on the non central atoms
what is the speed of light
c = 2.998 × 108 ms-1
equation for speed of light
c = fƛ
c = speed of light
ƛ = wavelength (m)
f = frequency (Hz or s-1)
what is the photoelectric effect
when light hits a metal surface, electrons can be ejected and you can measure their kinetic energy
what happens if light behaves like a wave
increasing intensity of light, increases energy of electrons
changing frequency or wavelength of light has no effect on energy
how to calculate energy of a photon with frequency
E = hf = hc/ƛ
h = 6.626 × 10-34 J
what is planck’s constant
h = 6.626 × 10-34 J
how electrons behave
act like particles or waves
when electrons fired at gold film, causes an electron diffraction pattern, which can only be explained by assuming electrons are waves
how to calculate De Broglie wavelength of a particle moving with speed v
ƛ = h / mv
ƛ = wavelength
h = planks constant
m = mass
v = speed
what does energy being quantised mean
there is only a certain set of possibilities: discrete set of energy levels (the change between them = quanta)
how to move a molecules energy up or down
absorption or emission of light can move up from the ground state or back down
draw out what happens when a photon is emitted
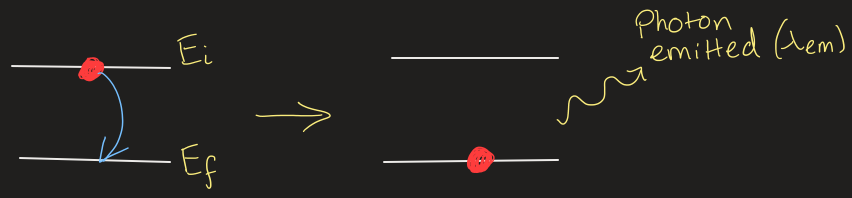
equation to calculate wavelength of photon emitted
|Ef - Ei| = hf = hc / ƛem
Ei = initial energy
Ef = final energy
ƛem = wavelength of photon emitted
(same for absorbed)
draw what happens when a photon gets absorbed by an atom
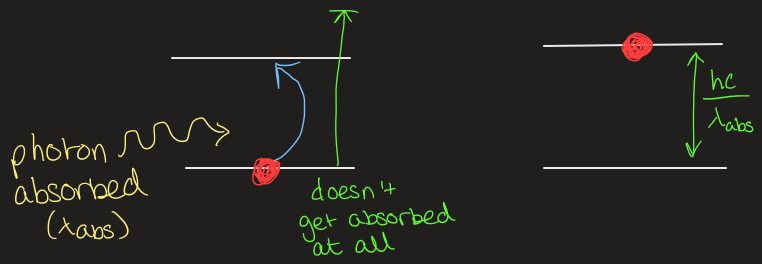
what can you see in atom emission spectra
certain frequencies
each line is a different emission wavelength of a photon
what is the Rydberg equation
1/ƛ = RH (1/n12 - 1/n22)
RH = 109,677.581 cm-1
n1, n2 = integers where n2 > n1
same as E = hc/ƛ = E(n1) - E(n2)
what did Schrödinger suggest
the electron in a hydrogen atom is described by a wave function that must obey an equation:
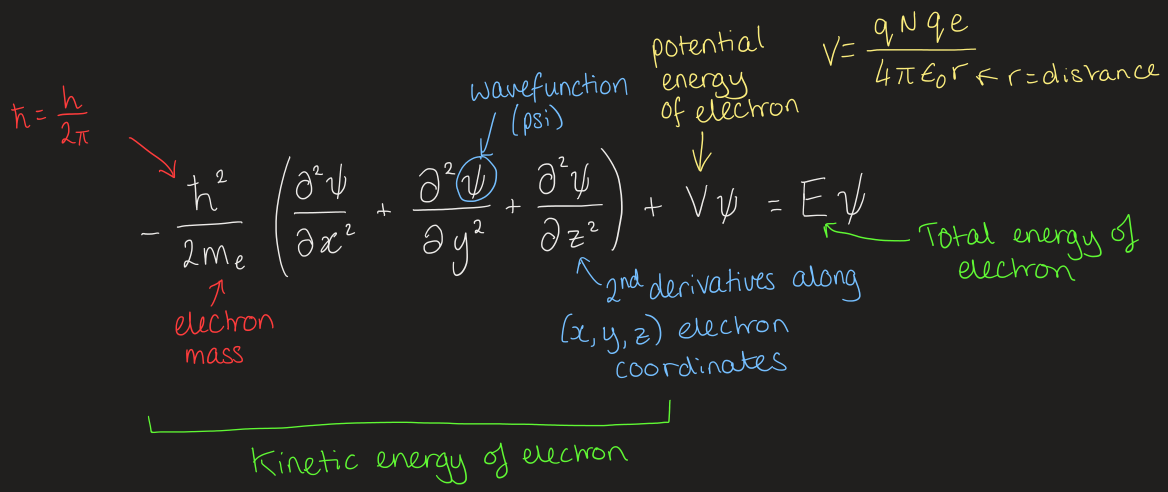
what does the wave function depend on
(x, y, z) coordinates of the electron
every electron position has a corresponding wave function value
what does schrodingers equation tell us
energy is quantised as only some wave functions obey the equation so there must be discrete energy levels
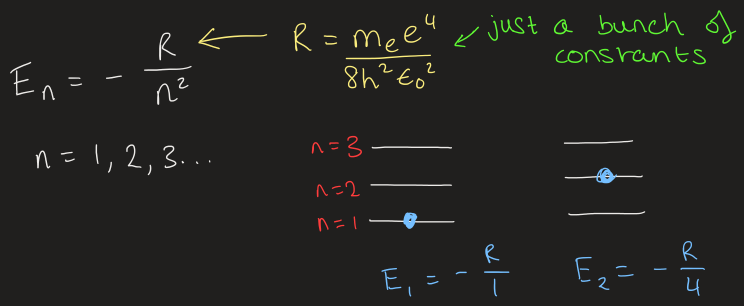
what are quantum numbers
the shapes of the allowed wave functions are characterised by 3 quantum numbers
name the 3 different quantum numbers and what they describe
principle quantum number
n
n = 1, 2, 3…
Same as the energy
i.e. in 1s, n=1 vs in 2p, n=2
angular quantum number
l
l = 0, 1, …, n-1
Same as the shape
i.e. in 1s, l represents the s and l=0
In 2p, l represents the p and l=1
magnetic quantum number
ml
ml = -l, -l-1, …, 0, …, l-1, l
Represents orientation
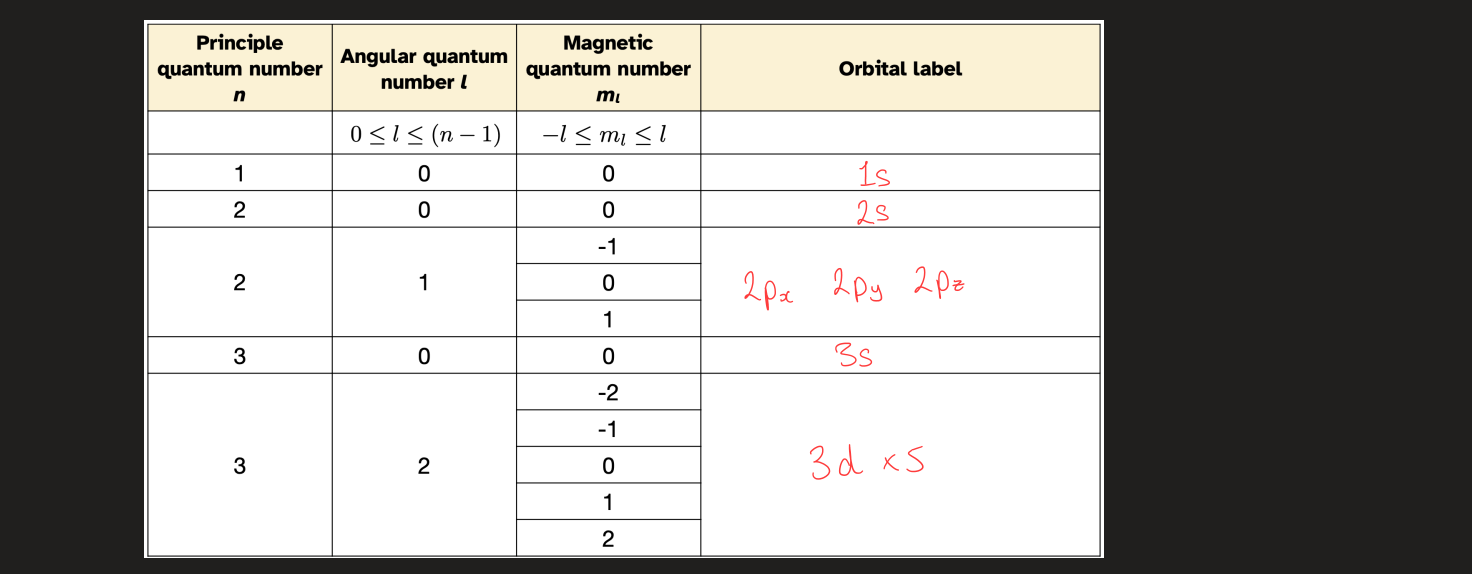
what do radial parts graphs show
wave function decays to 0 as we move away from the nucleus
shows where the electron likes to sit
have n - 1 - l radial nodes
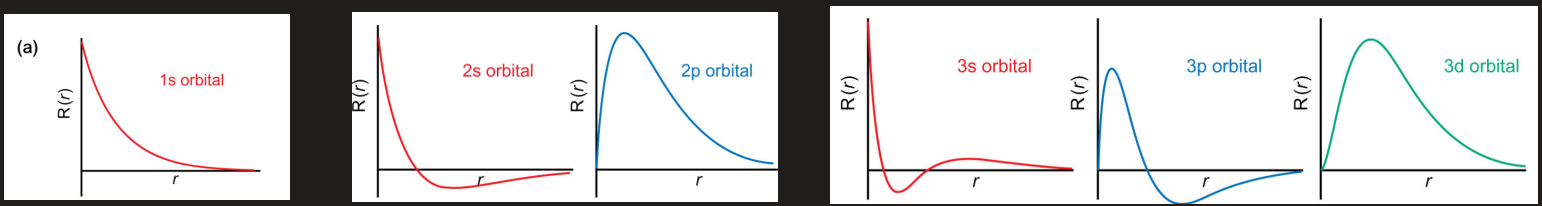
what does radial nodes tell about the shape of an orbital
the wave function is 0 so the number of radial nodes = number of nodal planes where electron density is 0
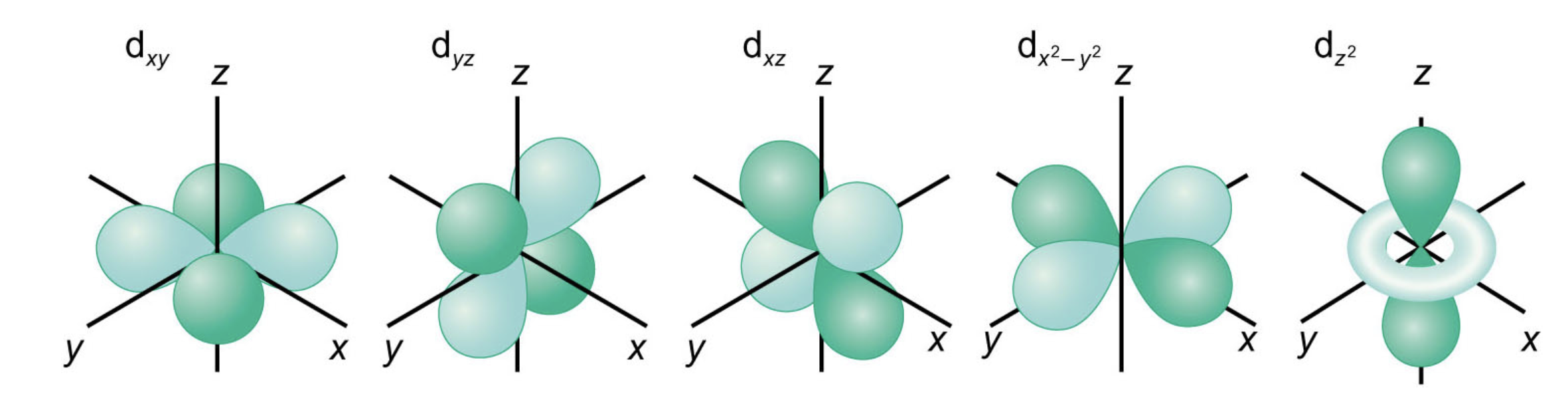
what do the 3d orbitals look like

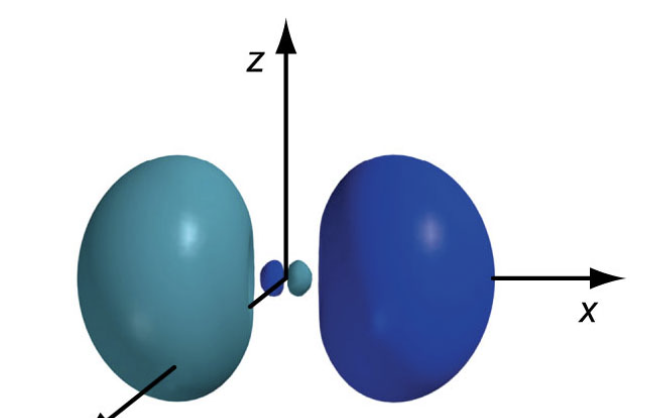
what do the 3p orbitals look like
why can schrodingers equation not be used for atoms with many electrons
repulsion between electrons makes solving it impossible but can still use atomic orbitals to explain structure/properties of many electron atoms
what is electron spin
an intrinsic property (cannot remove/destroy it)
spin quantum number = ms can be +1/2 or -1/2
what is the Pauli exclusion principle
no 2 electrons can occupy the same orbital and spin state
no 2 electrons can have the same set of 4 quantum numbers
can only have 2 electrons per orbital
what is the Aufbau principle
when placing electrons in atomic orbitals, you fill from the lowest energy and continue upwards
molecules tend to adopt their lowest electron configuration
changes in orbital penetration and shielding makes it more complex for many electron atoms as energy ordering of atomic orbitals changes
what is Hunds rule
electrons fill degenerate orbitals to maximise the number of electrons of parallel spin as electrons with parallel spins repel each other less
what does the wave function tell us
the born interpretation: the value of |ψ|2 tells us the probability of finding an electron at that point (electron density)
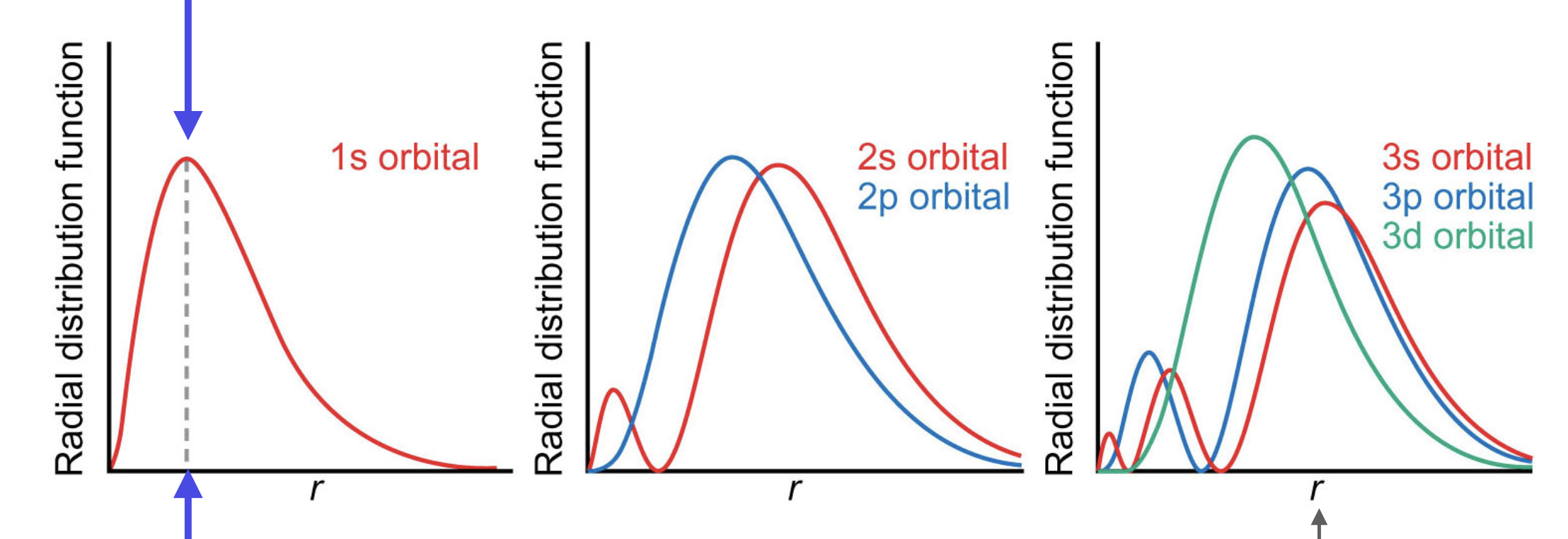
what do radial distribution functions tell us
the probability of seeing an electron anywhere on a shell at distance r from the nucleus
draw radial distribution functions for 1s, 2s+2p, and 3s+3p+3d orbitals
how does shielding effect the electrons in 2s/2p orbitals
1s electrons shield attraction between nucleus and electrons
effective nuclear charge is less than actual nuclear charge
how does penetration affect 2s and 2p electrons
2s electrons penetrate 1s orbital (less so for 2p orbital)
2s feels greater effective nuclear charge than 2p so 2s is more stabilised
2s electrons close to nucleus
energy of 2s is lower than 2p so it can be closer to the nucleus
equation for effective nuclear charge
Zeff = Z - S
Zeff = effective nuclear charge
Z = actual nuclear charge
S = shielding constant
what affects effective nuclear charge
electrons added to a shell increases Zeff
Zeff drops when electrons go into a shell with new principle quantum number due to shielding
trends in atomic radii and why
increase down the groups
outermost electrons sit in larger orbitals
maximum in radial distribution function moves outward as n increases
decrease across the periods
Zeff increases so electrons are drawn closer to nucleus
ionisation energy definition
energy required to remove an electron from an atom (endothermic)
first and second ionisation energy equations
A (g) → A+(g) + e-
A+(g) → A2+(g) + e-
how does ionisation energy change for each subsequent ionisation
energy required is greater as removing an electron from a charged species is harder than removing one from a neutral species
how does ionisation energy change across a period/down a group
across a period, ionisation energy increases with some dips
second period ionisation energies are higher than third periods ionisation energies dow to more tightly bound electrons
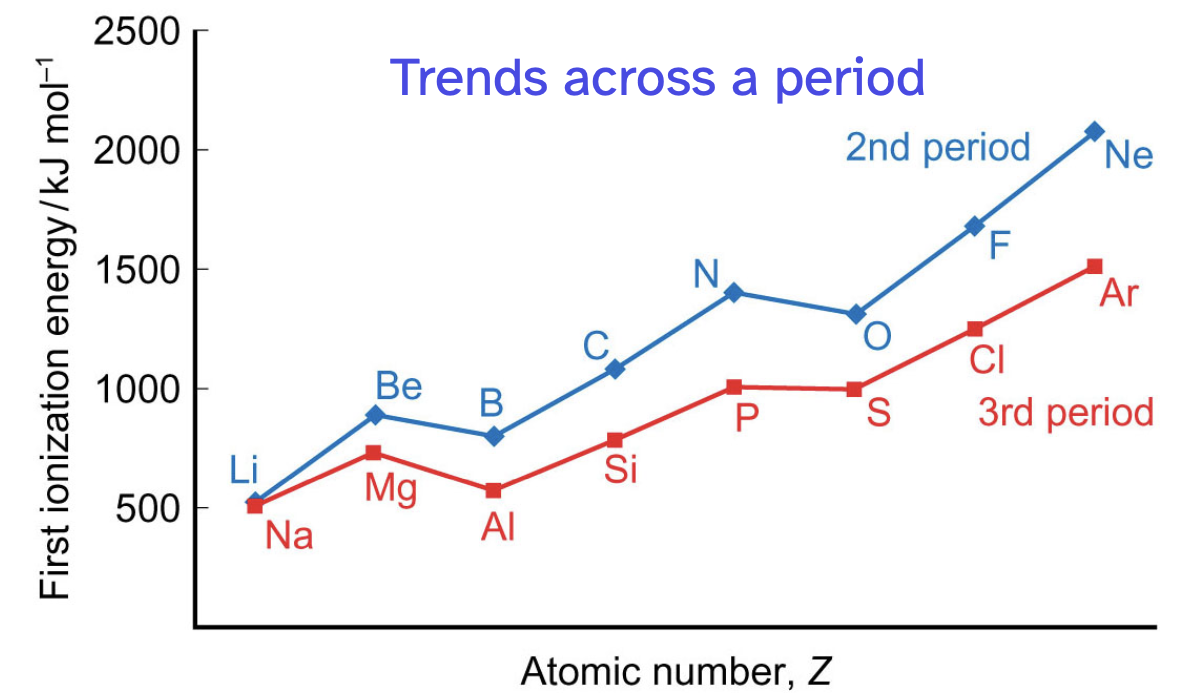
down a group, ionisation energy decreases as atoms get larger
electron gain energy definition
energy required to add an electron to an atom or ion
equations for first and second electron energy gain
A(g) + e- → A-(g)
A-(g) + e- → A2-(g)
when is electron gaining favourable
when electron gain energy value is negative
electronegativity trends
increases across a period as Zeff increases
decreases down a group as size increases
what is the LCAO approximation
approximate that MOs are a linear combination of atomic orbitals
AOs represent one-electron wave functions (have wave characteristics)
combine consecutively or destructively
what does it mean if AOs combine destructively
anti bonding occurs
how many MOs form from n AOs
n
what do the different symbols stand for in 1σg or 1σu*
1 = lowest energy σg or σu MO
σ = MO is spherically symmetric when viewed down inter-nuclear axis
g = MO looks same after inversion
u = MO changes sign after inversion
* = anti-bonding
draw MOELD for Li2
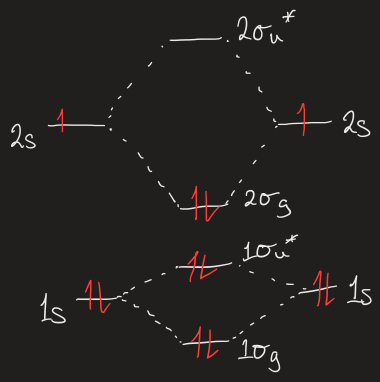
bond order equation
Bond order = 1/2[(electron count for bonding MOs) - (electron count for antibonding MOs)]
what is Born-Oppenheimer approximation
electrons are much lighter than nuclei so when a nuclei changes position, electrons instantaneously rearrange themselves into lowest energy MO
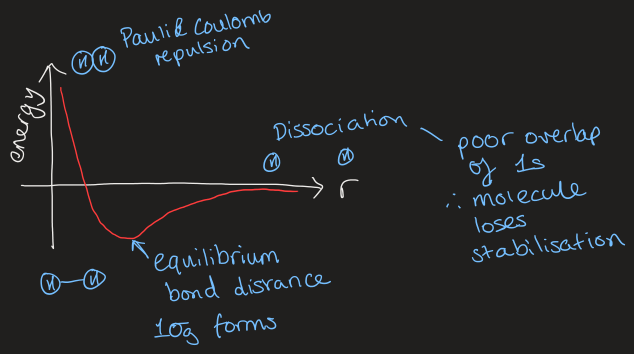
general rule for MOs
only AOs of the correct symmetry will interact to give MOs