proteins
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What elements do proteins contain?
Carbon, hydrogen, oxygen, nitrogen + sometimes sulfur
Define amino acids
The sub units (monomers) from which proteins (polymers) are formed
What is the general structure of an animo acid?
COOH - carboxylic acid group
R side group (represents the rest of the molecule)
NH₂ - amine group
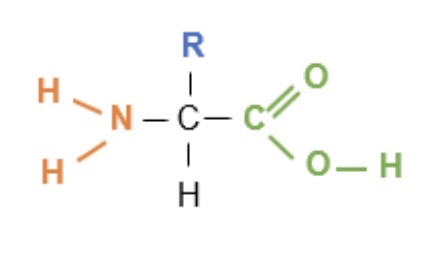
How many commonly occurring amino acids is there?
20 different ones
What makes the 20 amino acids different?
All amino acids have the same amine group + carboxylic acid group but what makes them different is there carbon-containing side / R groups which give them their different properties
Give some examples of properties of amino acids that their R group give them
Acidic (-ve charge)
Basic (+ve charge)
Polar (hydrophilic)
Non-polar (hydrophobic)
* +ve and -ve side chains can be attracted to each other
* hydrophobic groups attract each other + repel water
How are amino acids joined together?
By peptide bonds (-CONH-) which are formed during a condensation reaction, eliminating a molecule of water
→ 2 amino acids form a dipeptide
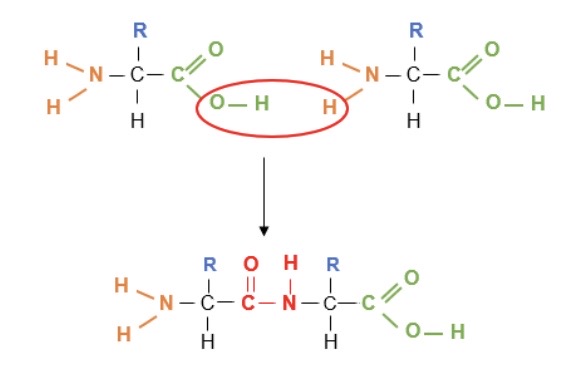
What reaction splits a dipeptide apart?
Hydrolysis
What is a polypeptide?
Many amino acids joined together in a chain
A functional protein may consist of one or more ____
polypeptide
How can proteins be hydrolysed?
By heating with acid
Using enzymes → proteases
Proteins vary in the ___, ___ and ____ of amino acids they contain → this produces a vast number of different protein molecules + are classified according to their ____
number, type and sequence
structure
Define the primary structure of proteins
It refers to the sequence of amino acids in the polypeptide chain → this sequence determines the specific shape of the protein
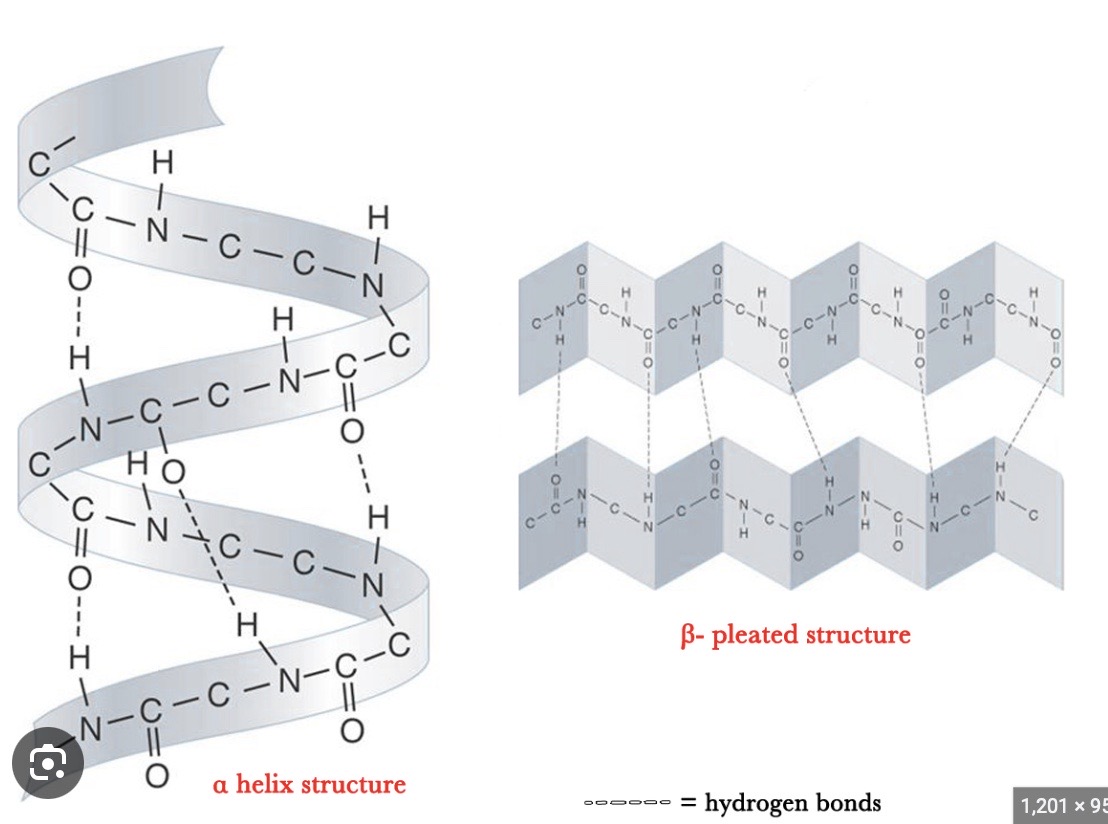
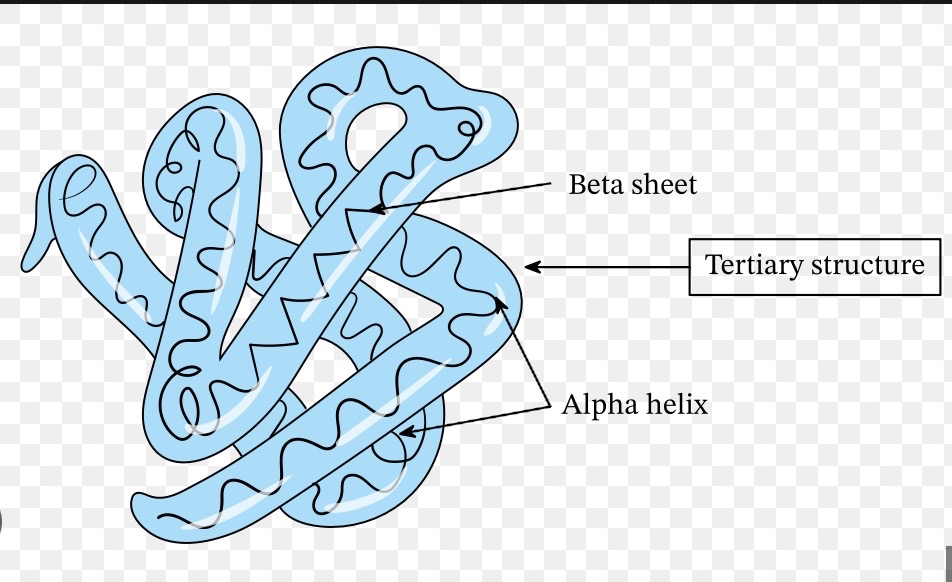
What does the secondary structure represent?
The folding / coiling of the polypeptide chain as a result of hydrogen bonding between the amino acids → secondary structures include the alpha helix + beta pleated sheet
ALPHA HELIX
all N-H bonds on same side of protein chain
spiral shape
H-bonds parallel to helical axis
BETA PLEATED SHEET
N-H + C=O groups alternate from one side to another
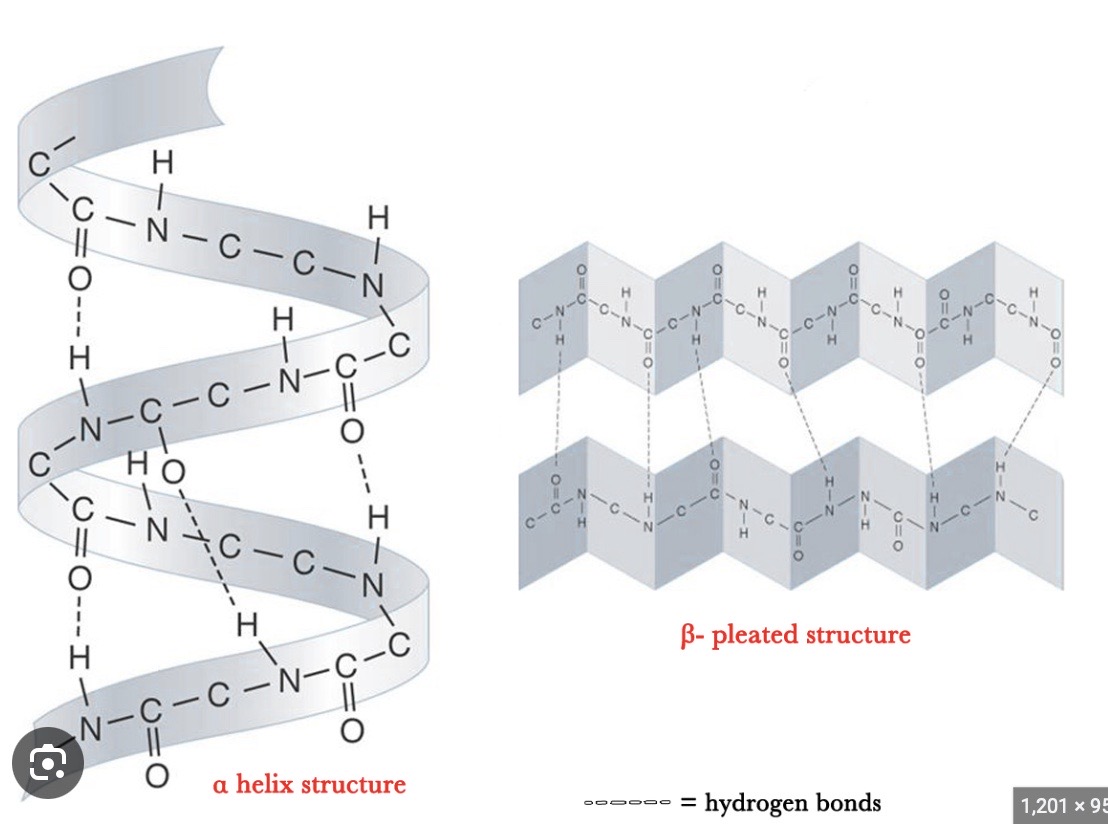
What is the tertiary structure formed by?
Further folding + coiling of the secondary structure due to hydrogen bonds, ionic bonds + disulphide bonds → these bonds form in places determined by the sequence of amino acids in the primary structure, they form between R groups of amino acids
Why do proteins have such a wide variety of roles?
Because tertiary structures are specific + unique e.g tertiary structure of an enzyme determines the shape of it active site + its precise function
TERTIARY STRUCTURE
Where do hydrogen bonds form?
Ionic bonds form between what?
Why do hydrophobic regions form?
Disulphide bond form as a result of what?
Hydrogen - between R groups of polar amino acids
Ionic - between positive + negatively charged side chain of basic + acidic amino acids respectively
Hydrophobic - because hydrophobic side chains exclude water + are attracted to each other
Disulphide - an oxidation reaction between two cysteines
What are globular proteins?
Soluble proteins that consist of a highly folded + coiled polypeptide chain to produce a compact, complex tertiary structure → include enzymes + antibodies
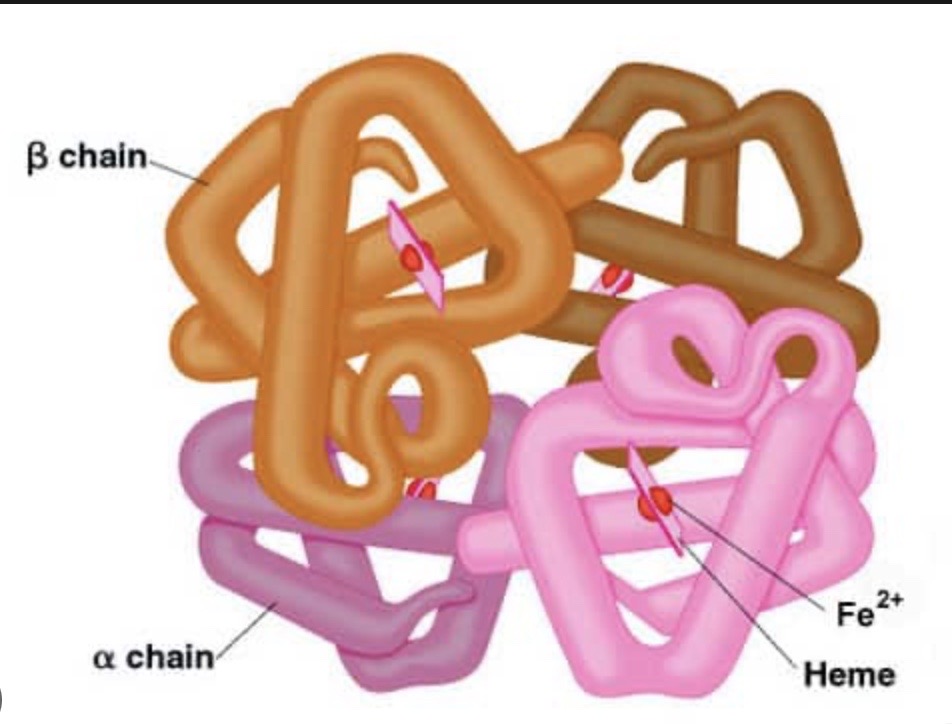
What is quaternary structure?
When proteins consist of more than one polypeptide chain
The polypeptide chains are held together by ionic, hydrogen and sometimes disulphide bonds
Can have non-protein groups involved in the quaternary structures
How can a small change in primary structure lead to a non-functional protein?
Change in sequence of amino acids means H-bonds form in different places, altering secondary structure → further H-bonds, ionic bonds, disulphide bridges form in different places → different tertiary structure
* if this protein is an enzyme : shape of active site is altered → no longer complementary to substrate → cant catalyse reactions
Define denaturation
An alteration in the tertiary structure of a protein → this loss of a 3-dimensional shape of the protein is often irreversible + protein is no longer functional
What can denaturation be caused by?
Breaking of hydrogen + ionic bonds
High temperatures above optimum → break H-bonds
Extreme changes in pH → break ionic bonds
Heavy metals → bind to a site on protein, changing its shape
Detergents → disrupts hydrophobic interactions
Reducing agents → breaks disulphide bridges
What bonds aren’t broken at the temperature that break hydrogen + ionic bonds?
Disulphide bonds
* In some proteins, disulphide bonds can remain unbroken at 70C
How to test a sample for protein
Add Biuret reagent to sample
A purple / lilac colour indicates protein is present
If solution remains blue, no protein is present