10.1 Electrolysis
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Define electrolysis
decomposition of an ionic compound using an electric current
During electrolysis:
> e- move in external c__
> ions move in e__
> OILRIG occurs
circuit, electrolyte
Define electrode
rod of metal/graphite where electric current flows into/out of electrolyte
Define electrolyte
ionic compound in molten/dissolved soln that conducts electricity
Define anode
+ve electrode
Define anion. What is it attracted to?
-ve charged ion, anode
Define cathode
-ve electrode
Define cation. What is it attracted to?
+ve charged ion, cathode
Acronym for which OILRIG at which electrode
red cat an ox
Soln type | Anode (+ve electrode) | Cathode (-ve electrode) |
Molten | __ (-ve ion) | __ (+ve ion) |
non-metal, metal
Soln type | Anode (+ve electrode) | Cathode (-ve electrode) |
Aq | If halogen: Ø Conc.: __ formed Ø Dilute: __ formed | If below H: Ø __ formed Ø e.g. Cu, Ag, Au |
If not halogen (e.g. SO42-): Ø __ formed | If above H: Ø __ formed |
halogen, o2, metal, o2, h2
Electrolysis of Lead Bromide
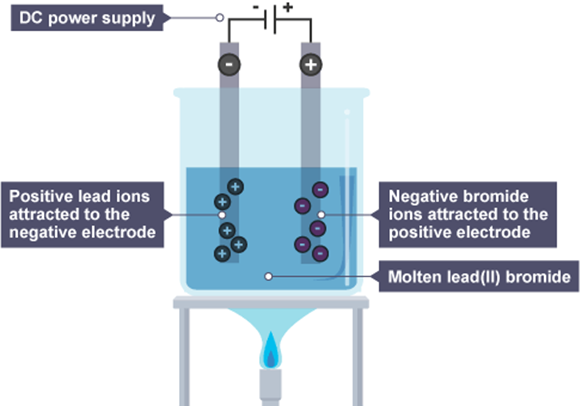
What is the ionic half eq at the -ve cathode (reduction)?
Pb2+ + 2e- -> Pb
Electrolysis of Lead Bromide

What is the ionic half eq at the +ve anode (oxidation)?
2Br- -> Br2 + 2e-
After electrolysis, O2 was formed. (i.e. in aq, dilute halogen OR no halogen)
Write the ionic half eq for the anion (at +ve anode; 2H2O →...)
2H2O -> O2 + 4H+ + 4e-
Electrolysis of b__ (conc. NaCl[aq])
> method: d__ c__
>> made of a__ & polymers
Write the ionic half eq at the -ve cathode
brine, diaphragm cell, abestos, 2H+ + 2e- -> H2
Uses of chlorine
> P__, w__ (pool), C2 solvents, etc
PVC, water
Uses of NaOH
> Na salts, s__, organics, etc
soap
O2 : H2 ratio in electrolysis of dilute H2SO4
1:2