haemostasis part 2: coagulation
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
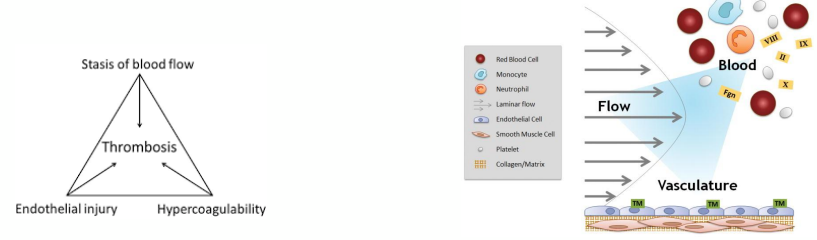
virchow’s triad
Described in 1850’s as the explanation for pathological thrombus formation
• Endothelial injury/dysfunction
• Stasis of blood flow in veins
• Change in coagulability of the blood
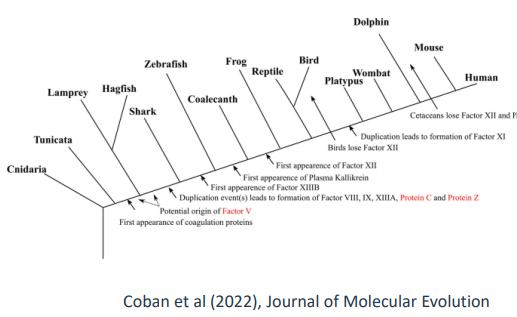
conservation of blood clotting
• Analysis of the coagulation factor network in bony fish (teleosts) suggests
it evolved over 430 million years ago
• Highly conserved in all vertebrates
• Very little change in the composition over time
• Positive: similarities mean that human medicine advances benefit veterinary
species
how we name coagulation factors - nomenclature
• Coagulation factors are identified by roman numerals
• Labelled as F for factor eg factor 8 is FVIII
• This refers to a zymogen – inactive precursor (also called a procofactor)
• Active form of enzyme has a lower case a after name eg FVIIIa
• Inhibited (deactivated) forms have a lower case i eg FVIIIai
Some factors are referred to by name instead:
• Tissue factor (TF) = FIII = thromboplastin
• Prothrombin (PT) = FII -cleaved to give us thrombin
• Thrombin = FIIa

recurring themes across the network
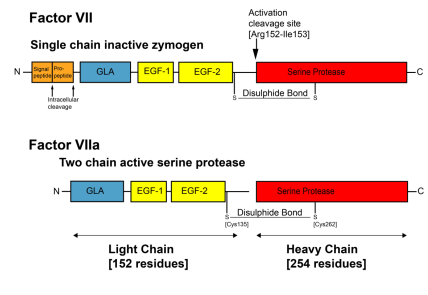
• Coagulation factors are mostly proteases
• Specifically, serine proteases of the chymotrypsin super famil
• They circulate as zymogens that are activated by proteolysis - (single to light and heavy chain)
• For maximal activity they require cofactors
• The cofactors also circulate in plasma in procofactors that are also activated by cleavage
• The complexes assemble on –ve charged phospholipid surfaces on platelets
clotting factor production
• Factors mainly synthesised in the liver
• Circulate in inactive form
• Most are serine proteases except TF, FV, FVIII (all glycoproteins) and FXIII (transglutaminase)
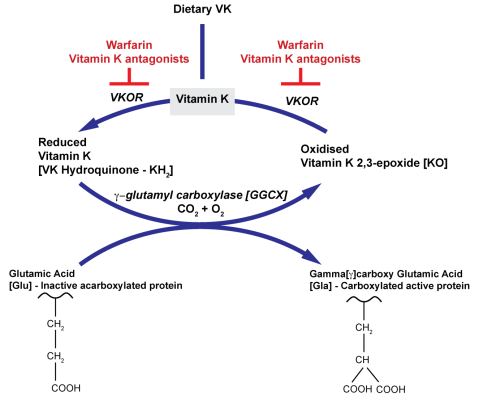
• Vitamin K (fat soluble vitamin found in leafy greens) is an essential factor for
gamma -glutamyl carboxylase which adds a carboxyl group to glutamic acid (Gla)
residues on FII, FVII, FIX and FX
• Vitamin K is regenerated by VKOR (Vit K epoxide reductase)
• VKOR is a target of warfarin
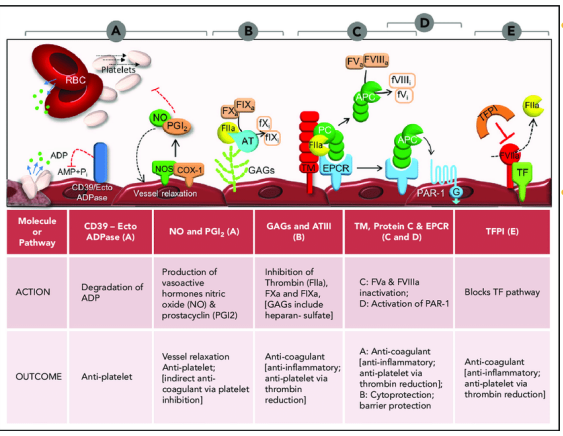
T H E R E S T I N G S T A T E
• Tissue factor (FIII) – trigger of blood coagulation (extrinsic pathway) is
expressed on surface of cells not ordinarily in contact with the blood –
this is termed the Haemostatic envelope
• Endothelial cells present an anticoagulant surface by expression of
cell surface anticoagulant proteins
• Thrombomodulin (TM)
• Tissue factor pathway inhibitor (TFPI)
• Antithrombin (AT)
• Heparin sulphate proteoglycans
I N I T I A T I O N O F T H E P R O C O A G U L A N T R E S P O N S E
1. Vascular damage exposes liquid blood to cells expressing TF
2. TF associates with blood FVIIa and this TF:FVIIa complex activates FIX (to FIXa) and FX (to FXa) (extrinsic pathway)
3. FXa coverts prothrombin to thrombin (common pathway)
4. Thrombin cleaves (soluble) fibrinogen to (insoluble) fibrin to promote clot stabilisation
• Amplification network causes explosive thrombin generation (RAPID)
• Thrombin activates platelets and activated platelets drive thrombin generation on
procoagulant surface
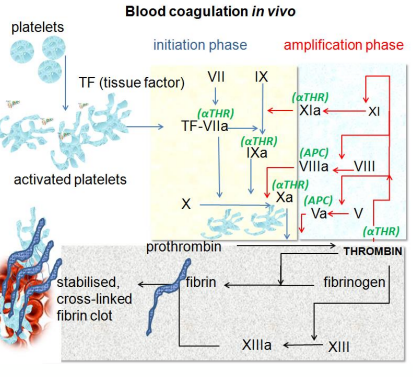
I N I T I A T I O N O F T H E P R O C O A G U L A N T R E S P O N S E - 2
• Coagulation process is delicately balanced with central feature being conversion of fibrinogen to fibrin
• To be effective FIXa and FXa require assembly into complexes with non-enzymatic cofactors
• FVa
• FVIIIa ( role in haemophillia)
• Cofactors circulate as inactive form (activated by thrombin)
• Back activation of these cofactors occurs from trace amounts of thrombin made in the initiation phase
• This cofactor activation supports the explosive burst of thrombin generation (referred to as Tenase because of role in making more FX)
• This complex also assembles on phospholipid or fibrin clot surfaces
A M P L I F I C A T I O N P R O C E S S
• Cofactor binding amplifies the activity of the individual protein
• TF:FVIIa complex is 103 fold more active than FVIIa alone
• FXa:FVa complex is 105 to 106 fold more active than FXa alone
• FIXa:FVIIIa complex is 103 fold more active than FIXa alone
• Thrombin generated also activates more platelets increasing the procoagulant surface
• Thrombin can activate more FXI leading to more FIX
• More fibrin = more procoagulant surface
• Thrombin activates FV, FVIII and their inhibitor protein C
• FXIIIa is important for crosslinking fibrin – stabilising function
T H E F U L L C O A G U L A T I O N C A S C A D E
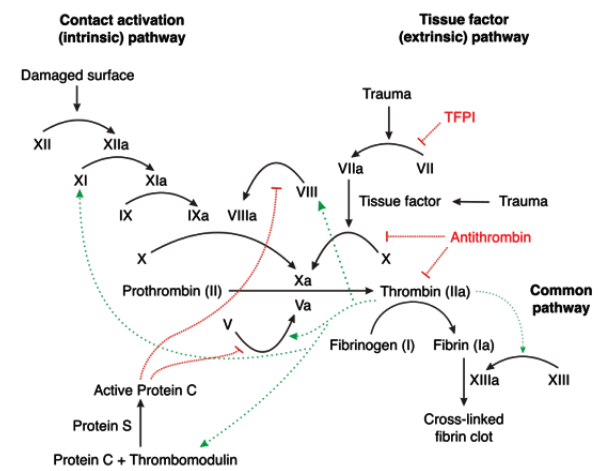
H O W I S C O A G U L A T I O N K E P T L O C A L I S E D ?
• Platelets and coagulation factors react to proteins exposed only by vascular injury
• Activated platelets locally provide a negatively charged procoagulant surface
• Thrombin generation is triggered by TF exposure that is normally hidden under the
endothelium as part of the haemostatic envelope
• Undisturbed endothelium releases/has surface bound anticoagulant factors to dampen inappropriate activation
• This also stops the thrombus from spreading – otherwise blood becomes jelly!
H O W I S I T K E P T S O C O N T R O L L E D ?
Anticoagulant feedback mechanisms limit extent of activation
• Initiation complex is shut down
• TFPI inactivates initiation complex by complexing TF:FVIIa:FXa:TFPI
• TFPI release from EC is stimulated by thrombin
• Reducing the cofactor effectiveness
• Thrombin near the EC surface binds TM activating the PC anticoagulant pathway
• Proteolysis/activation of FV, FVIII and fibrin is not effective with thrombin:TM complex
• aPC in complex with cofactor protein S rapidly inactivates FVa and FVIIIa by proteolysis
• Limiting fibrin conversion by reducing the activity of serine proteases
• AT inhibits FIXa, FXa, FXIa and thrombin
• Accelerated by heparin sulphate proteoglycans on surface of EC
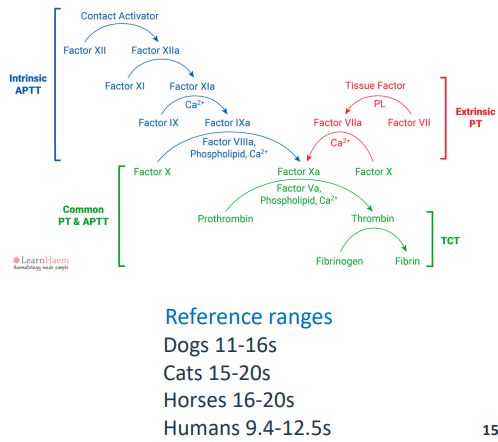
L A B O R A T O R Y T E S T I N G - APTT
• Activated partial thromboplastin time
• Blood collected into an anticoagulant called citrate (to chelate calcium)
• Plasma prepared by centrifugation
• Initiate coagulation (Kaolin or silica(charge activator) + calcium)
• Measure time to form a fibrin clot
• Appropriate reference plasma needed (same species, at least 8 animals)
• Testing for deficiencies in the intrinsic pathway
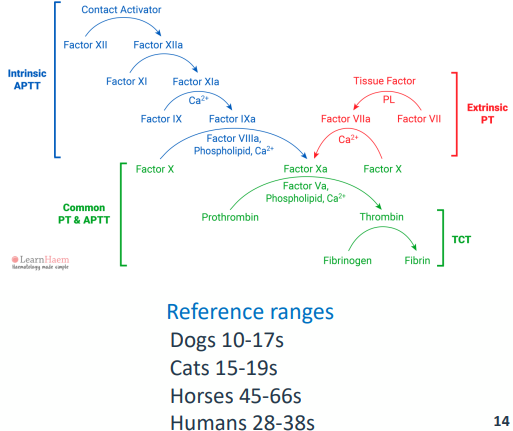
L A B O R A T O R Y T E S T I N G - PT
• Prothrombin time
• Blood sample collected in citrate
• Plasma prepared by centrifugation
• Coagulation initiated by TF
• + added calcium
• Time to formation of fibrin clot measured
• Reference plasma from 8 animals
• Important to pick correct TF
• Testing for deficiencies in the extrinsic pathway
H A E M O P H I L L I A A/B I N H U M A N S A N D A N I M A L S
• 2 bleeding disorders with identical clinical presentation
• Lab testing required to distinguish A from B
• In humans 400K people worldwide living with haemophilia
• Only 25% getting adequate treatment
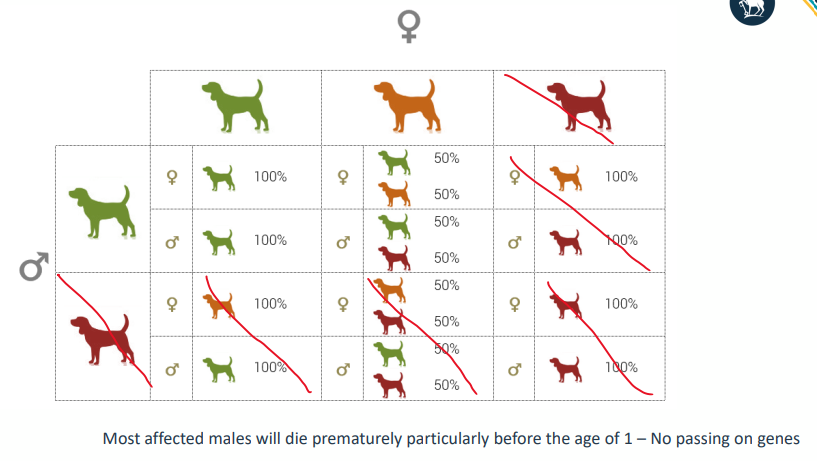
Haemophillia A
• 1:10K births (1:5K male births) mostly spontaneous mutations
• Deficiency in FVIII
• X chromosome location
Haemophilia B
• 1:50K births
• Deficiency in FIX
• Also on X chromosome
• Both disorders are described in dogs and cats
• Haemophilia A is more common in animals as well as humans
HAEMOPHILLIA A/B
• Most severe inherited coagulation disorder
• Diagnosed by APTT test with FVIII specific controls, pedigree analysis
• Gene on X chromosome gets a spontaneous mutation which can persist across many generations
• Presence of 1 normal gene is enough to prevent haemophilia
• Animals have spontaneous bleeding into joints and muscles
• Bleeding under skin and from gums
• Risk of haemorrhage in surgery
• Severity and survival depends on the mutation
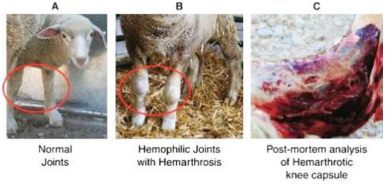
I N H E R I T A N C E O F H A E M O P H I L L I A
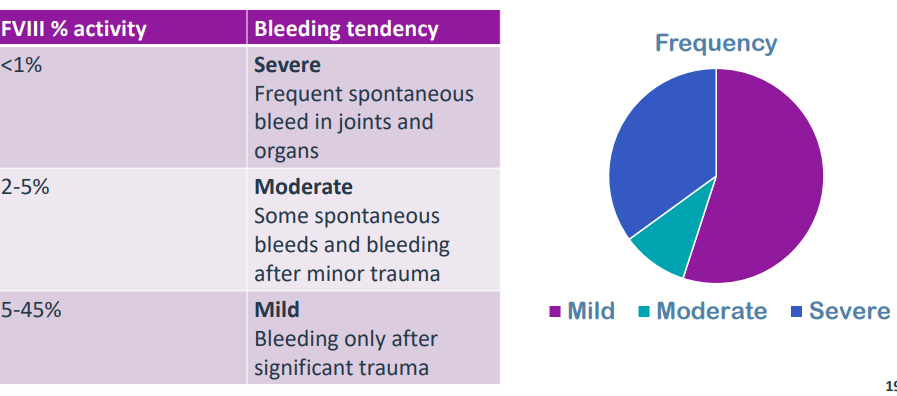
C L I N I C A L S E V E R I T Y O F H A E M O P H I L L I A A
Divided into 3 severities depending on the amount of FVIII activity
M O L E C U L A R G E N E T I C S A N D O T H E R D I S O R D E R S
• Wide range of variants causing haemophilia A
• 3052 reported in 10144 humans
• 50% missense, 24% deletion, rest made up of nonsense, insertion, splice, duplication and indel
• FIX mutations also numerous (1244 in 4713 individuals)
• Haemophilia A/B most common disorder
• FXI disorder -autosomal inheritance, mild bleeding
• FVII disorder is rare – autosomal inheritance, causes cerebral haemorrhages at birth
• FVII deficiency is found in ~30% of beagles (not X linked, autosomal, carriers not affected but close family breeding increases inheritance )
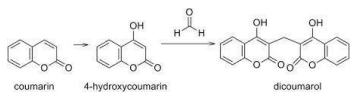
A C Q U I R E D B L E E D I N G : S W E E T C L O V E R P O I S O N I N G
• Caused by feeding mouldy sweet clover hay or silage that contains dicoumarol
• Dicoumarol is a fungal metabolite of coumarin in sweet clover
• Toxin interferes with synthesis and metabolism of vitamin K
• Vitamin K required for some clotting factors production
• Causes bleeding and liver damage
• Mostly affecting cattle and some horses
• Testing using PT
• Can be treated with blood transfusion or synthetic vitamin K

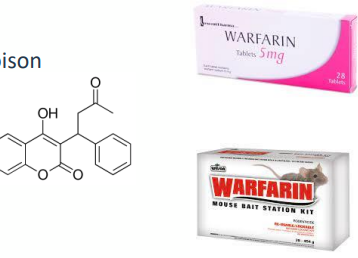
W A R F A R I N P O I S O N I N G
• Warfarin is used clinically in humans as cheap treatment for DVT, PE or stroke
• Requires careful monitoring to prevent bleeding
• Interferes with vitamin K conversion by inhibiting VKOR
• Impact on FII, FVII, FIX, FX production
• Coumarin derivative related to sweet clover poison
• Frequently used as a rodenticide
• Not species specific though
• Relatively common poison of cats and dogs
• Death due to haemorrhage
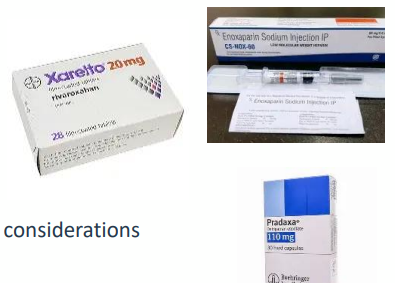
A N T I C O A G U L A N T D R U G S
• Warfarin –vitamin K dependent clotting factor production inhibitor
• Heparin - activates antithrombin, rapid anticoagulation in emergencies
• Low molecular weight heparins e.g enoxaparin – also activates antithrombin
• Used in hypertrophic cardiomyopathy/heart conditions which are risks for coagulation
• Factor Xa inhibitors e.g rivaroxiban
• Routinely used for humans
• Direct oral anticoagulant
• Fewer drug/diet interactions
• Thrombin inhibitors e.g. dabigatran
• Rare in veterinary practice
• Costs/ monitoring and route of administration are considerations
T R E A T I N G B L O O D L O S S
Saline/salt solutions can be used to expand blood volume (<1.5L lost)
• Will dilute coagulation proteins though
Fresh frozen plasma
• Replaces coagulation factors lost
Platelets - Packed red cells- Whole blood but with most of the plasma and leukocytes removed
Replacement coagulation factors
• Useful for congenital deficiencies
Activated coagulation factors
• FVIIa or thrombin to help support coagulation
Fibrinolysis inhibitors
• Tranexamic acid – increases clot strength by antifibrinolytic action
• Useful in greyhounds who are more likely to develop trauma /surgical induced delayed bleeding