Animal physiology
0.0(0)
0.0(0)
Card Sorting
1/395
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
396 Terms
1
New cards
Tissues
Specialized cells rarely function alone, but rather as groups of these imilar cells. There are many different kinds of cells, but they are all classified as one of four these groups types: **epithelial**, **muscle**, **connective**, or **nervous**.
2
New cards
To survive, organisms must:
* extract energy and nutrients from the environment
* build all internal structures they need
* eliminate toxins and metabolic waste products
* sense the environment and respond to it in various ways, including movement
* maintain constant conditions in their internal environments
* reproduce
* build all internal structures they need
* eliminate toxins and metabolic waste products
* sense the environment and respond to it in various ways, including movement
* maintain constant conditions in their internal environments
* reproduce
3
New cards
BMR measured when:
1. In thermoneutral zone (range of temp. where MR is minimal)
2. Fasting (no more SDA)
3. Resting
4
New cards
Basal metabolic rate (BMR)
Applies to homeotherms;The metabolic rate of a resting endotherm at a temperature within its thermoneutral zone.
5
New cards
Standard metabolic rate (SMR)
Applies to poikilotherms (ectotherms).
6
New cards
SMR measured when:
1. Fasting (no more SDA)
2. Resting
i.e., SMR varies with temp.
7
New cards
Epithelial tissues
Are sheets of cells that create barriers between different compartments and frequently have secretory functions.
8
New cards
Muscle tissues
Contract to generate forces and movement.
9
New cards
Connective tissues
Provide structure and support.
10
New cards
Nervous tissues
Convey and process information.
11
New cards
Organ system
A group of organs that work together to carry out certain functions.
12
New cards
Blood plasma
Is the fluid portion of the blood and is 20% of all extracellular fluid.
13
New cards
Interstitial fluid
Is the fluid that batches the cells of the body and is 80% of all extracellular fluid.
14
New cards
Homeostasis
A narrow range of stable and optimal physical and biochemical conditions.
15
New cards
Set point
The desired temperature.
16
New cards
Comparator
Senses the current temperature and compares that value to the set point. Thus the sensing of the temperature is **feedback** information.
17
New cards
Error signal
The results of any difference between the set point and feedback information; is converted into commands.
18
New cards
Regulatory systems
They obtain, process, and integrate information; they use that information to issue commands to **effectors** such as muscles or glands that effect changes in the internal environment. Effectors are also called **controlled** **systems** because their activities are controlled by the neural or hormonal signals from their respective -. Important components of any - are the sensors such as light-, temperature-, and pressure-sensitive cells that provide feedback information to be compared with internal set points.
19
New cards
Negative feedback
Is information used to counteract the influence that created an error signal.
20
New cards
Positive feedback
Amplifies a response.
21
New cards
Feedforward information
Is a feature of regulatory systems that changes the set point in anticipation of a change in conditions.
22
New cards
Thermoregulatory adaptations
Enables animals to tolerate extreme conditions or to control their body temperatures in spite of environmental conditions in order to stay within thermal limits for optimal function.
23
New cards
Temperature sensitivity (Q10)
This of a reaction or process can be described in terms of Q10, a factor calculated by dividing the rate of a process or reaction at a certain temperature, RT, by the rate of that same process or reaction at a temperature 10 degrees Celsius lower, RT–10:
Q10 = (RT)/(RT-10)
Q10 = (RT)/(RT-10)
24
New cards
Acclimatized
To (cause to) change to suit different conditions of life, weather, etc.
25
New cards
Isozymes
Are enzymes that differ in amino acid sequence but catalyse the same chemical reaction.
26
New cards
Homeotherms
Animals that maintain a constant body temperature.
27
New cards
Poikilotherms
Animals that experience a fluctuating body temperature.
28
New cards
Endotherms
Have the ability to vary their metabolic heat production to compensate for the loss of heat to the environment.
29
New cards
Ectotherms
Are largely dependent on environmental sources of heat.
30
New cards
Heterotherms
Organisms that act like ectotherms some of the time and like endotherms at other times.
31
New cards
Radiation
Heat moves from warmer objects to cooler ones via the exchange of infrared -.
32
New cards
Convection
Heat exchanges with a surrounding medium such as air or water that flows over a surface.
33
New cards
Conduction
Heat flows directly between two objects at different temperatures when they come into contact.
34
New cards
Evaporation
Heat is transferred away from a surface when water evaporates on that surface.
35
New cards
Energy budget
The total balance of heat production and heat exchange, based on the simple fact that if the body temperature of an animal is to remain constant, the heat entering the animal must equal the heat leaving it. The heat coming in is usually from metabolism and radiation (Rabs, for radiation absorbed). Heat leaves the body via the four mechanisms listed above—radiation emitted (Rout), convection, conduction, and evaporation. The - takes the mathematical form:
Heat in = heat out
Heat in = heat out
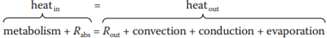
36
New cards
Aorta
A large blood vessel with oxygenated blood.
37
New cards
Countercurrent heat exchange
Heat is exchanged between blood vessels carrying blood in opposite directions.
38
New cards
Thermoneutral zone
When within a narrow range of environmental temperatures, the metabolic rates of endotherms (birds and mammals) are at low levels and independent of environmental temperature. It is bounded by a **lower critical temperature** and an **upper critical temperature**.
39
New cards
Brown fat
Most nonshivering heat production occurs in specialized adipose tissue. In these cells, a protein called **thermogenin** uncouples proton movement from ATP production, allowing protons to leak across the inner mitochondrial membrane rather than having to pass through the ATP synthase and generate ATP. In nonshivering thermogenesis, metabolic fuels are consumed without producing ATP, but heat is still released.
40
New cards
Hypothalamus
The major thermoregulatory integrative centre of mammals at the base of the brain. This centre is a key player in many regulatory systems of vertebrates.
41
New cards
Hypothermia
Is a below-normal body temperature.
42
New cards
Daily torpor
Daily bouts of regulated hypothermia.
43
New cards
Hibernation
Regulated hypothermia that lasts for days or even weeks, during which the body temperature falls close to environmental temperature.
44
New cards
Respiratory gases
That what animals must exchange are oxygen (O2 ) and carbon dioxide (CO2 ). Cells need to obtain O2 from the environment to produce an adequate supply of ATP by cellular respiration. CO2 is an end-product of cellular respiration, and it must be removed from the body to prevent toxic effects.
45
New cards
Ventilation
Mechanisms that move air or water over the environmental sides of those surfaces.
46
New cards
Perfusion
Mechanisms that circulate extracellular fluids on the internal sides.
47
New cards
Partial pressures
Describes the concentrations of different gases in a mixture.
48
New cards
Fick’s law of diffusion
Whether in air or water, the diffusion rates of respiratory gases between an animal and its respiratory medium—air or water— depend on the partial pressure gradients across the gas exchange surfaces and on other factors that are described quantitatively with a simple equation:
* Q is the rate at which a gas such as O2 diffuses between two locations.
* D is the diffusion coefficient, which is a characteristic of the diffusing substance, the medium, and the temperature.
* A is the area across which the gas is diffusing.
* P1 and P2 are the partial pressures of the gas at the two locations.
* L is the path length, or distance, between the two locations.
* (P1 – P2 )/L is a partial pressure gradient.
* Q is the rate at which a gas such as O2 diffuses between two locations.
* D is the diffusion coefficient, which is a characteristic of the diffusing substance, the medium, and the temperature.
* A is the area across which the gas is diffusing.
* P1 and P2 are the partial pressures of the gas at the two locations.
* L is the path length, or distance, between the two locations.
* (P1 – P2 )/L is a partial pressure gradient.
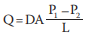
49
New cards
External gills
Gills are highly branched and folded extensions of the body surface that provide a large surface area for gas exchange. These gills are vulnerable to damage and are tempting morsels for predators.
50
New cards
Internal gills
Protective body cavities for gills.
51
New cards
Lungs
Are highly divided internal cavities with large surface areas for respiratory gas exchange with air. Because it’s tissue is elastic, it can be inflated with air and deflated as a means of ventilating the respiratory exchange surfaces.
52
New cards
Tracheae
A respiratory gas exchange system consisting of a network of air-filled tubes in insects that branch through all tissues of the insect’s body. It divides into two smaller airways, the **primary bronchi** (singular bronchus). The primary bronchi extend all the way to the posterior air sacs and also branch into **secondary** **bronchi**. The posterior air sacs also have connections to the secondary bronchi. Secondary bronchi divide into tubelike **parabronchi** that run parallel to one another through the lungs in a posterior to anterior direction.
53
New cards
Afferent blood vessels
Bring deoxygenated blood to the gills.
54
New cards
Efferent blood vessels
Take oxygenated blood away from the gills.
55
New cards
Countercurrent blood flow
Blood flows through the lamellae in the opposite direction to the flow of water over the lamellae. This flow maximizes the transfer of O2 from water to blood.
56
New cards
Dead space
The air remaining in lungs and airways after exhalation.
57
New cards
Air sacs
Are an important and unique feature of the avian respiratory system, and they occupy much of the body cavity of the bird. They can be divided into a group of anterior air sacs and a group of posterior air sacs. The are interconnected with each other, with the lungs, and with air spaces in some of the bones. They are not gas exchange surfaces; rather, they act as bellows to maintain a unidirectional flow of air through the lungs.
58
New cards
Pharynx
Where air that enters the lungs through the oral cavity or through the nasal passages join together.
59
New cards
Larynx
Or the voice box, which houses the vocal cords.
60
New cards
Bronchi
Are the major air passageways of the lungs. They lead to the **bronchioles**, which are finely branched, as are the blood vessels.
61
New cards
Alveoli
Are the sites of gas exchange.
62
New cards
Surface tension
Gives the surface of a liquid the properties of an elastic membrane.
63
New cards
Surfactant
Is a substance that reduces the surface tension of a liquid.
64
New cards
LaPlace’s law
Which describes the relationships between pressure (P), tension (T), and radius (r) of bubbles: P = 2T/r. The larger the radius, the less pressure it takes to overcome the tension. Alveoli are not all the same size, so according to , inhalation should preferentially inflate the larger alveoli and not the smaller ones. The unique property of lung surfactant solves this problem because as the larger alveoli inflate, their surface tension increases, favouring the inflation of the smaller alveoli. Also, the increased surface tension in the inflated lungs adds to the recoil during exhalation, facilitating the emptying of the lungs.
65
New cards
Thoracic cavity
A closed compartment bounded on the bottom by a sheet of muscle called the **diaphragm**.
66
New cards
Pleural membrane
A continuous sheet of tissue that covers each lung and also lines the thoracic cavity adjacent to the lung.
67
New cards
Intercostal muscles
Between the ribs are two sets of these muscles. The external - muscles expand the thoracic cavity by lifting the ribs up and outward. The internal - muscles decrease the volume of the thoracic cavity by pulling the ribs down and inward. During strenuous exercise, the external - muscles increase the volume of air inhaled, making use of the inspiratory reserve volume, and the internal - muscles increase the amount of air exhaled, making use of the expiratory reserve volume. The abdominal muscles can also aid in breathing. When they contract, they cause the abdominal contents to push up on the diaphragm and thereby contribute to the expiratory reserve volume.
68
New cards
Hematocrit
The percent of the blood volume consisting of RBCs.
69
New cards
Positive cooperativity
The influence of O2 binding to one Hgb subunit on the O2 affinity of the other subunits.
70
New cards
Myoglobin
Consists of just one polypeptide chain associated with an iron-containing ring structure that can bind one O2 molecule. It has a higher affinity for O2 than Hgb does, so it picks up and holds O2 at PO2 values at which Hgb is releasing its bound O2. It facilitates the diffusion of O2 in muscle cells and provides an O2 reserve for times when metabolic demands are high and blood flow is interrupted.
71
New cards
Bohr effect
The influence of pH on the function of Hgb.
72
New cards
Carbonic anhydrase (CA)
Speeds up the conversion of CO2 to H2CO3.
73
New cards
Carotid and aortic bodies
They are chemosensors. In these nodes of neural tissue we have physiological sensitivity to blood PO2 on the large blood vessels leaving the heart.
74
New cards
Circulatory system
Consists of a muscular pump (heart), a fluid (blood), and a series of conduits (blood vessels) through which the fluid is pumped throughout the body.
75
New cards
Cardiovascular system
Heart, blood, and vessels.
76
New cards
Gastrovascular system
Highly branched central cavities that bring the external environment into the animal.
77
New cards
Heats
Muscular chambers in circulatory systems that move the extracellular fluid through the body.
78
New cards
Hemolymph
The extracellular fluid that is the same as the fluid in the circulatory system, in open circulatory systems.
79
New cards
Extracellular fluid
In animals with a closed circulatory system, it refers to both the fluid in the circulatory system and the fluid outside it.
80
New cards
Interstitial fluid
The extracellular fluid outside the circulatory system.
81
New cards
Open circulatory systems
In these systems a heart moves the hemolymph through vessels leading to different regions of the body. The fluid leaves the vessels to filter through the tissues before returning to the heart.
82
New cards
Ostia
Opening through where the fluid returns directly to the heart. They have valves that allow hemolymph to enter the relaxed heart but prevent it from flowing in the reverse direction when the heart contracts.
83
New cards
Arteries
Blood flows out of the heart and into these vessels that carry blood away from the heart.
84
New cards
Capillaries
Are tiny, thin-walled vessels where materials are exchanged between blood and the interstitial fluid.
85
New cards
Arterioles
Smaller branches from arteries that feed blood into capillary beds.
86
New cards
Venules
Small vessels that drain capillary beds.
87
New cards
Veins
Consists out of venules that ultimately deliver blood back to the heart.
88
New cards
Pulmonary circuit
When blood is pumped from the heart to the lungs and back to the heart.
89
New cards
Systemic circuit
When blood is pumped from the heart to the rest of the body and back to the heart.
90
New cards
Sinus venosus
Where blood returning from all parts of the body collects, that feeds into the muscular **atrium**.
91
New cards
Atrium
Pumps blood into the more muscular chamber, the **ventricle**.
92
New cards
Bulbus arteriosus
The last chamber where contraction of the ventricle pushes blood into. It is a highly elastic chamber.
93
New cards
Aorta
A large dorsal artery where blood leaving the gills collects in, which distributes blood to smaller arteries and arterioles leading to all the organs and tissues of the body.
94
New cards
Atrioventricular (AV) valves
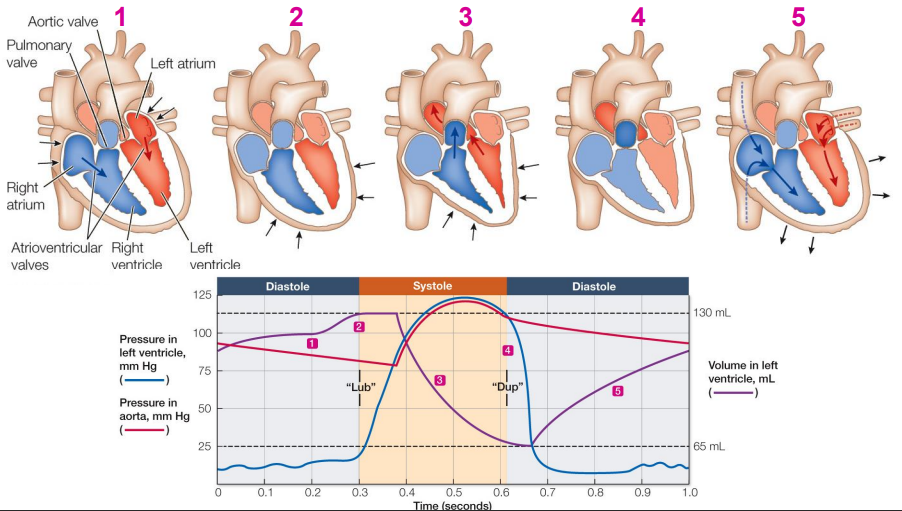
One-way valves between the atria and ventricles, prevent backflow of blood into the atria when the ventricles contract. The right - valve is called the **tricuspid valve** because it has three leaves. The left AV valve is called the **bicuspid valve** because it has two leaves. The bicuspid valve is also called the **mitral valve** because it has the shape of a religious headdress called a miter. There are also one-way valves between the ventricles and the arteries leaving the heart. The **pulmonary valve** goes to the lungs, and the **aortic valve** goes to the aorta. These two valves are also called **semilunar** **valves** because their separate leaves are shaped like half-moons.
95
New cards
Superior (upper) vena cava and the inferior (lower) vena cava
Are the large veins that return deoxygenated blood from the upper and lower body to the right atrium.
96
New cards
Pulmonary artery
Where the right ventricle pumps the blood into when it contracts, causing the tricuspid valve to close.
97
New cards
Pulmonary veins
Return the oxygenated blood from the lungs to the left atrium, from where the blood enters the left ventricle through the bicuspid valve.
98
New cards
Cardiac cycle
Contraction of the two atria, followed by contraction of the two ventricles and then relaxation. It is divided into two phases: **systole**, when the ventricles contract, and **diastole**, when the ventricle relax. At the very end of diastole, just before the ventricles contract, the atria contract and top off the volume of blood in the ventricles. The sounds of the —the “lub-dup” heard through a stethoscope—are created by the heart valves slamming shut.
99
New cards
Pacemaker cells
Some cardiac muscle cells that initiate action potentials without stimulation from the nervous system. These specialized muscle cells do not contract, but they generate rhythmic sequences of action potentials that spread to neighbouring cardiac muscle cells that do contract. Since cardiac muscle cells are in electrical contact with one another through gap junctions, the action potentials from the pacemaker cells spread rapidly through a large mass of cardiac muscle, causing it to contract in unison. Coordinated contraction is essential for pumping blood effectively.
100
New cards
Sinoatrial node
The primary pacemaker of the heart is a group of modified cardiac muscle cells, located at the junction of the superior vena cava and right atrium.