CH. 2 Families of Carbon Compounds Lecture
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
Alkane
carbon and hydrogen single bonds
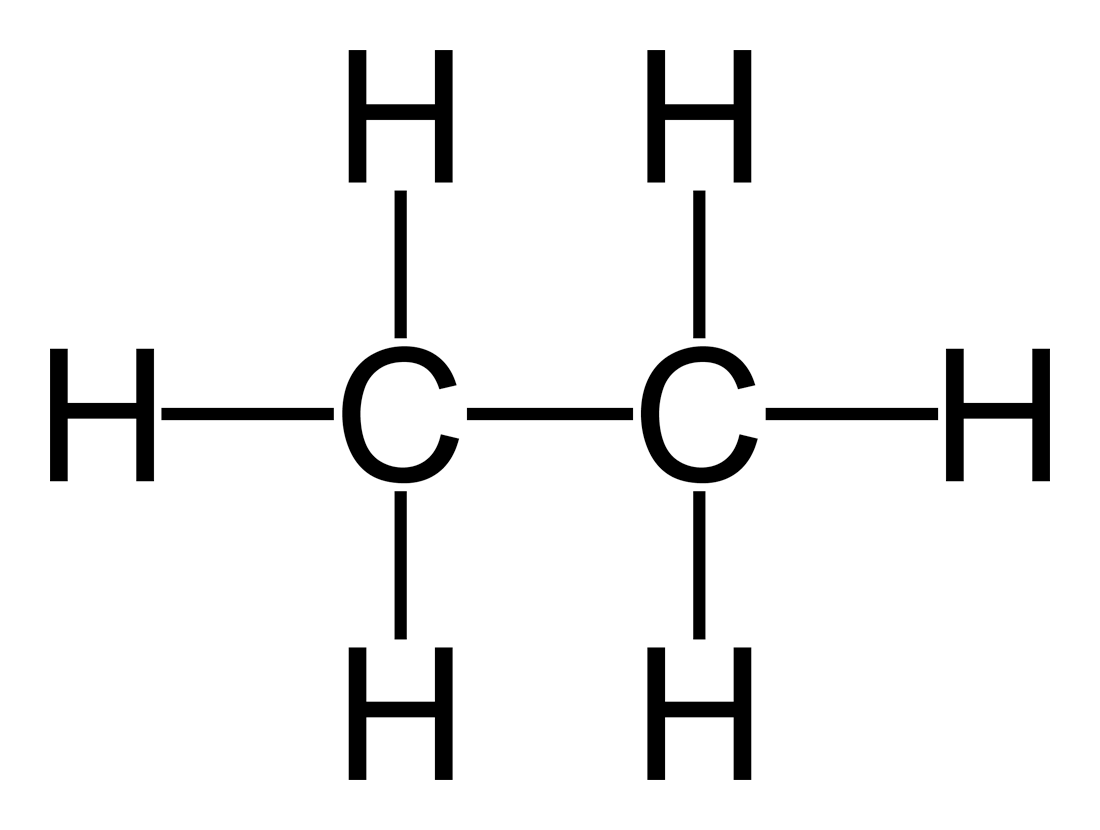
Alkene
carbon-carbon double bond
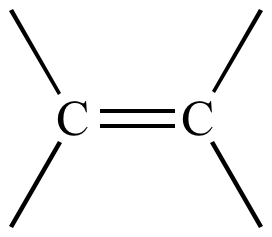
Alkyne
carbon-carbon triple bond
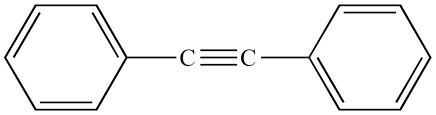
Arene/ Aromatic
six membered ring with alternating double and sing bonds
Each carbon is sp² hybridized
Phenyl group
benzyl group
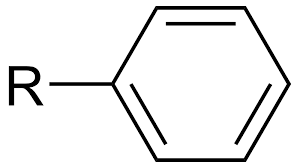
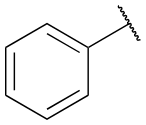
Phenyl group
benzene ring attached to another group
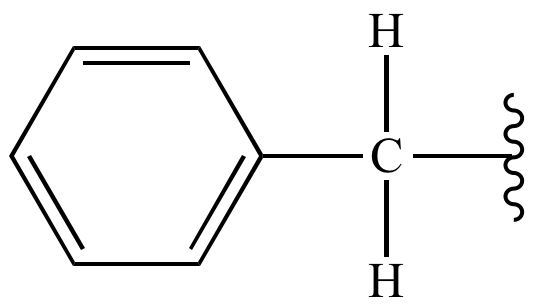
Benzyl group
phenyl and methylene (-CH2)
Polar Covalent Bonds
formed from atoms with different electronegativities
They have a dipole
Unit for dipole moment is debye (μ)
Polar and Nonpolar
Polar molecules have a dipole moment
Nonpolar molecules have no net dipole moment (but the bonds can be polar)
Cis/ trans isomers have different dipole moments
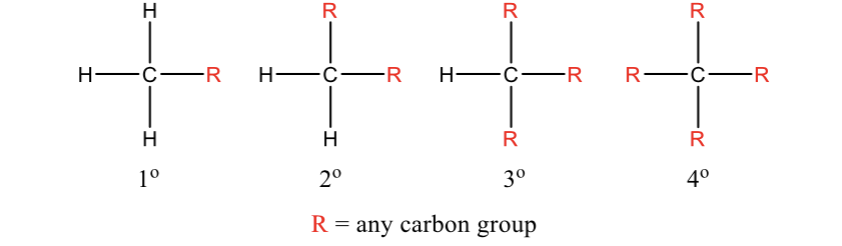
Types of Carbons
1) Primary (1o)- carbon attached to one other carbon
2) Secondary (2o)- carbon attached to two other carbons
3) Tertiary (3o)- carbon attached to three other carbons
4) Quaternary (4o)- carbon attached to four other carbons
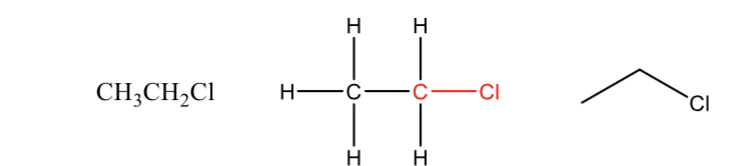
Alkyl Halides
a carbon bonded to a halogen like Cl, Br, I, or F
The carbon attached to halogen needs to be sp³
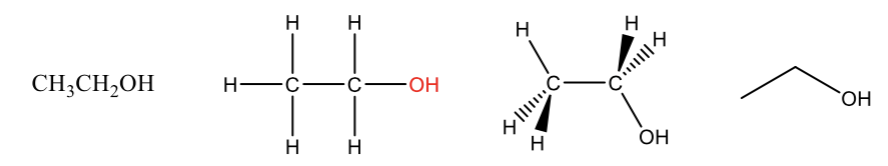
Alcohol
contains a (-OH) group (a hydroxyl group)
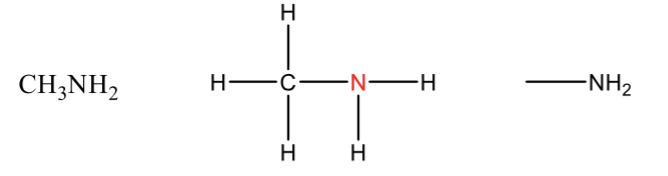
Amine
contains nitrogen usually bonded to a carbon group or hydrogen
Nitrogen is sp³
Tetrahedral arrangement of electrons
Trigonal pyramidal molecular geometry

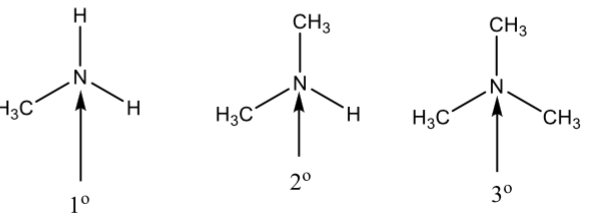
Types of Amines
based on the number of carbon groups attached tot the nitrogen
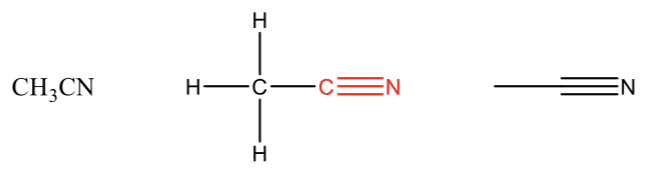
Nitrile
carbon triple bonded to nitrogen
Nitro
has a (-NO2) group
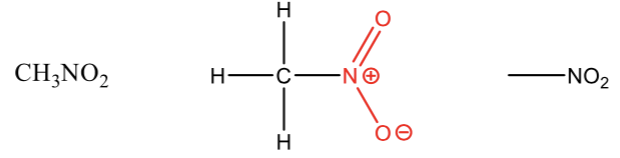
Ether
an oxygen atom between two carbons
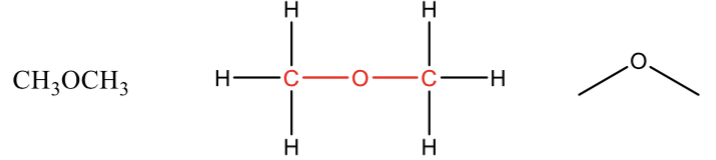
Acid chloride
carbonyl (C=O) bonded to a chlorine atom
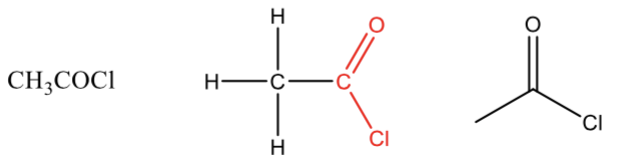
Aldehyde
a carbonyl (C=O) bonded to a hydrogen
Ketone
a carbonyl (C=O) bonded to two carbons
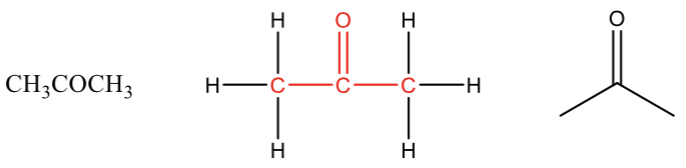
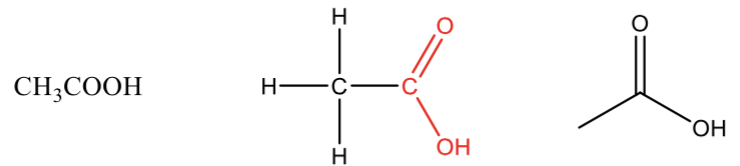
Carboxylic acid
a carbonyl (C=O) bonded to an -OH
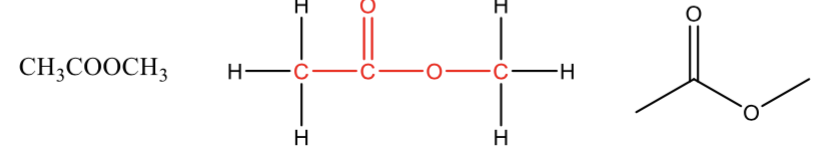
Ester
a carbonyl bonded to carbon and an oxygen with a carbon
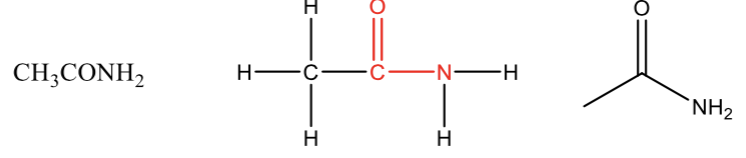
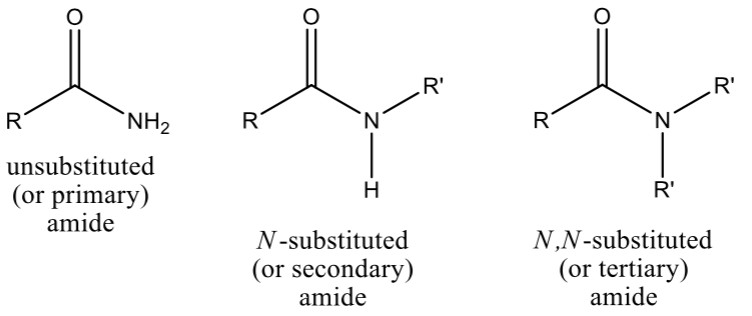
Amide
a carbonyl bonded to an amine
Type of Amides
based on carbon groups attached to nitrogen
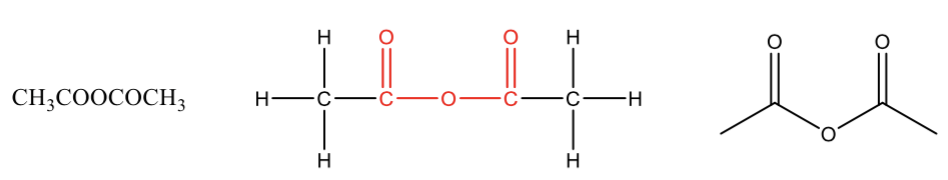
Carboxylic Acid Anhydride
contains two carbonyls linked through an oxygen
Functional Groups
most functional groups have a dipole
Can be represented by arrows
Can be represented by delta symbol
Physical Properties
can help identify substance
Melting point
boiling point
solubility
Ion-Ion Intermolecular Force
Holds ionic compounds together
Very strong
Ionic compounds have high melting point
Ex. NaCl
Dipole-Dipole Intermolecular Force
Permanent dipole moments
Forces hold most organic compounds together
Forces b/w polar compounds
Weak force, easy to break
Ex. Acetone, CH3COCH3
Hydrogen Bonds
Very strong dipole-dipole interaction
Occurs b/w O, N, or F (strong electronegative atoms) and a hydrogen attached to an O, N, or F
Weaker than ordinary covalent bonds but much stronger than dipole-dipole forces
Takes more energy to break H-bonds
Hydrogen Bonds
compounds that can form H-bonds have higher boiling point than compounds that cannot
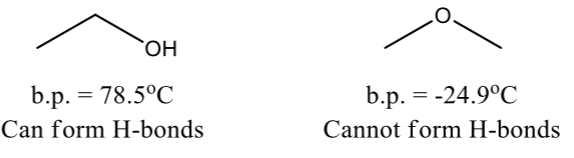
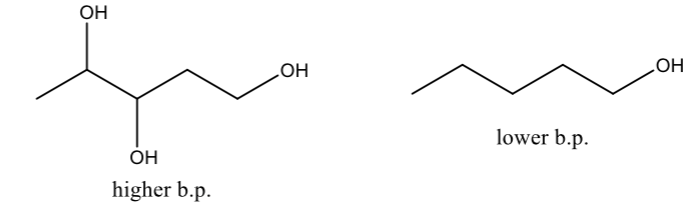
The more -OH groups, the higher the boiling point in compound with similar molecular weight and number of carbon
Hydrogen Bonds
Branching decreases the boiling point and melting point of compound
Less surface area and more compact structures lead to fewer attractions
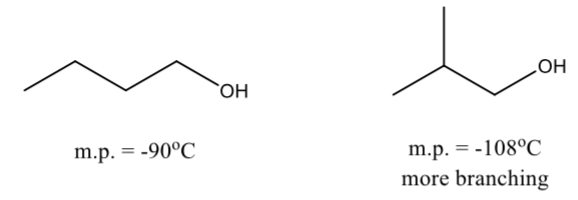
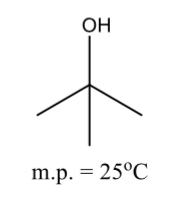
Symmetrical alcohols do not follow the general rule above
They have abnormally high melting point
Dispersion Forces
induced dipole or van de Waals or London forces
Temporary dipole results from the electrons in atoms moving within the atom
Molecules have an induced (+) and (-) end
Attractive forces b/w nonpolar molecules
What are the 2 important factors of determining the magnitude of dispersion forces?
1) Polarizability of electrons- how easily the electrons respond to a changing electric field
The larger the atom, the farther away its electrons are from the nucleus, the more polarizability (Cl<Br<I)
Atoms with unshared electrons are easily polarized compared to atoms with all bonding electrons.
2) Surface area- the larger the surface area, the larger the overall attraction between molecules
Surface Area
the larger the surface area, the larger the the overall attraction b/w molecules
Boiling Points
The temperature at which the vapor pressure of the liquid equals the vapor pressure of the atmosphere
boiling point is pressure dependent: decreasing the pressure, decreases the boiling point
Nonpolar compounds with weak forces usually boil at low temp.
Heavier molecules need more energy to boil (surface area is larger and so are the dispersion forces)
Solubility
“like dissolves like”
Nonpolar compounds dissolve in nonpolar solvents
Water hydrates or solvates ionic compounds
Alkyl groups are hydrophobic (water fearing)
-OH groups are hydrophilic (water loving)
Oil is not soluble in water- it is all hydrocarbon
The longer the hydrocarbon chain, the less water soluble
after the 5 carbon, compound becomes less soluble
Physical Properties of Alkanes
Boiling point of unbranched alkanes increase with molecular weight
Branching decreases surface area and decreases boiling point
Density: alkanes have very low densities and float on water
Alkanes are insoluble in water
Infrared Spectroscopy
can give you structural information about a compound
what FUNCTIONAL GROUP are in a compound
Type of electromagnetic radiation
Has lower energy than visible light
Infrared Spectroscopy
all molecules have molecular vibrations
when infrared radiation is absorbed, the frequency of the radiation matches the frequency of the molecular vibration
IR Spectrum
3000-3500: C-H, O-H, N-H
2200: C≡N; C≡C
1600-1800: C=O; C=N; C=C
Alkane
C-C
2850-2960
medium
Alkanyl
C-H
Less an 3000
Alkene
C=C
1640-1680
Medium
Alkenyl
=C-H
3020-3100
Medium
Alkyne
C≡C
2100-2260
Medium
Alkenyl
≡C-H
3300
Strong
Alcohol
O-H
3400-3650
Strong, broad
Arene
C-H
3030
weak
Aromatic Ring
1660-2000
weak
Amine
N-H
3300-3500
medium
Carbonyl Compound
C=O
1670-1780
Strong
Nitrile
C≡N
2210-2260
medium
Aldehyde
Fermi doublet
about 2800-2810
only have a fermi doublet
Double bond
1500-2000
Triple Bond
2000-2500